Kidney transplantation in highly sensitized recipients
- PMID: 34233438
- PMCID: PMC8476304
- DOI: 10.23876/j.krcp.21.012
Kidney transplantation in highly sensitized recipients
Abstract
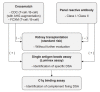
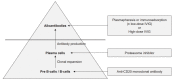
In kidney transplantation (KT), overcoming donor shortage is particularly challenging in patients with preexisting donor-specific antibodies (DSAs) against human leukocyte antigen (HLA), called HLA-incompatible KT (HLAi KT), carrying the risk of rejection and allograft loss. Thus, it is necessary to accurately evaluate the degree of sensitization before HLAi KT, and undertake appropriate pretreatment strategies. To determine the degree of sensitization, complement-dependent cytotoxicity has been the only method employed; the development of a method using flow cytometry further improved the test sensitivity. However, these tests present disadvantages, including the need for living cells, with a solid-phase assay developed to resolve this problem. Currently, the method using Luminex (Luminex Corp.) is widely used in clinical practice. As this method measures DSAs using single antigen beads, it is possible to classify immunological risks by measuring the type and amount of DSAs. Furthermore, there have been major advances in methods that involve DSA removal before HLAi KT. In the early stages of desensitization, plasmapheresis and intravenous immunoglobulins were the main treatment methods employed; however, the introduction of CD20 monoclonal antibody and proteasome inhibitors further increased the success rate of desensitization. Currently, HLAi KT has been established as an important transplant method, but an understanding of DSAs and a novel desensitization treatment are warranted.
Keywords: Alloantibodies; Bortezomib; HLA antigens; Kidney transplantation; Plasmapheresis; Rituximab.
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures


Similar articles
-
Excellent outcome after desensitization in high immunologic risk kidney transplantation.PLoS One. 2019 Sep 24;14(9):e0222537. doi: 10.1371/journal.pone.0222537. eCollection 2019. PLoS One. 2019. PMID: 31550258 Free PMC article.
-
The effect of desensitization therapy in kidney transplantation.Clin Exp Nephrol. 2018 Feb;22(1):179-187. doi: 10.1007/s10157-017-1424-7. Epub 2017 Jun 20. Clin Exp Nephrol. 2018. PMID: 28634772
-
The Impact of Donor-Specific Anti-Human Leukocyte Antigen (HLA) Antibody Rebound on the Risk of Antibody Mediated Rejection in Sensitized Kidney Transplant Recipients.Ann Transplant. 2017 Mar 28;22:166-176. doi: 10.12659/aot.902266. Ann Transplant. 2017. PMID: 28348361
-
Management of children undergoing cardiac transplantation with high Panel Reactive Antibodies.Cardiol Young. 2011 Dec;21 Suppl 2:124-32. doi: 10.1017/S1047951111001703. Cardiol Young. 2011. PMID: 22152539 Review.
-
Clinical and immunological relevance of antibodies in solid organ transplantation.Int J Immunogenet. 2016 Dec;43(6):351-368. doi: 10.1111/iji.12294. Int J Immunogenet. 2016. PMID: 27870356 Review.
Cited by
-
Differences between Very Highly Sensitized Kidney Transplant Recipients as Identified by Machine Learning Consensus Clustering.Medicina (Kaunas). 2023 May 18;59(5):977. doi: 10.3390/medicina59050977. Medicina (Kaunas). 2023. PMID: 37241209 Free PMC article.
-
Time-dependent impact of immunosuppressant regimens on cardiovascular outcomes in kidney transplant recipients: a nationwide cohort study.Front Pharmacol. 2025 May 13;16:1540576. doi: 10.3389/fphar.2025.1540576. eCollection 2025. Front Pharmacol. 2025. PMID: 40432895 Free PMC article.
-
Impact of sirolimus on long-term adverse cardiovascular outcomes in kidney transplant recipients: A nationwide cohort study.Eur J Clin Invest. 2025 Jul;55(7):e70027. doi: 10.1111/eci.70027. Epub 2025 Mar 19. Eur J Clin Invest. 2025. PMID: 40105194 Free PMC article.
-
Epidemiology, Clinical Manifestations, and Outcome of Mucormycosis in Solid Organ Transplant Recipients: A Systematic Review of Reported Cases.Open Forum Infect Dis. 2024 Jan 30;11(6):ofae043. doi: 10.1093/ofid/ofae043. eCollection 2024 Jun. Open Forum Infect Dis. 2024. PMID: 38887489 Free PMC article. Review.
-
Role of Complement-dependent Cytotoxicity Crossmatch and HLA Typing in Solid Organ Transplant.Rev Recent Clin Trials. 2024;19(1):34-52. doi: 10.2174/0115748871266738231218145616. Rev Recent Clin Trials. 2024. PMID: 38155466 Review.
References
-
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. - PubMed
-
- Satayathum S, Pisoni RL, McCullough KP, et al. Kidney transplantation and wait-listing rates from the international Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2005;68:330–337. - PubMed
-
- Korean Network for Organ Sharing (KONOS) Seoul (Korea): KONOS; c2020. KONOS annual report; updated September 8, 2020 [Internet] [cited 2020 Dec 11]. Available from: https://www.konos.go.kr/konosis/common/bizlogic.jsp.
-
- Kwon KH, Moon JI, Chang HJ, et al. The results of renal transplantation after lymphocyte cross-match negative conversion by combination therapy with plasmapheresis, intravenous gamma globulin and potent immunosuppresants in patients with positive LCM. J Korean Soc Transplant. 2002;16:172–177.
LinkOut - more resources
Full Text Sources
Research Materials

