Schwann cells promote prevascularization and osteogenesis of tissue-engineered bone via bone marrow mesenchymal stem cell-derived endothelial cells
- PMID: 34233721
- PMCID: PMC8261922
- DOI: 10.1186/s13287-021-02433-3
Schwann cells promote prevascularization and osteogenesis of tissue-engineered bone via bone marrow mesenchymal stem cell-derived endothelial cells
Abstract
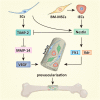
Background: Tissue-engineered bone grafts (TEBGs) that undergo vascularization and neurotization evolve into functioning bone tissue. Previously, we verified that implanting sensory nerve tracts into TEBGs promoted osteogenesis. However, the precise mechanisms and interaction between seed cells were not explored. In this study, we hypothesized that neurotization may influence the osteogenesis of TEBGs through vascularization.
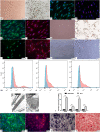
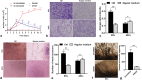
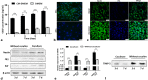
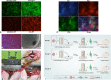
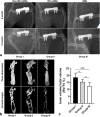
Methods: We cultured rat Schwann cells (SCs), aortic endothelial cells (AECs), and bone marrow-derived mesenchymal stem cells (BM-MSCs) and then obtained BM-MSC-derived induced endothelial cells (IECs) and induced osteoblasts (IOBs). IECs and AECs were cultured in an SC-conditioned medium (SC-CM) to assess proliferation, migration, capillary-like tube formation, and angiogenesis, and the vascular endothelial growth factor (VEGF) levels in the supernatants were detected. We established an indirect coculture model to detect the expression of nestin and VEGF receptors in IECs and tissue inhibitor of metalloproteinase (TIMP)-2 in SCs. Then, SCs, IECs, and IOBs were labeled and loaded into a β-tricalcium phosphate scaffold to induce prevascularization, and the scaffold was implanted into a 6-mm-long defect of rat femurs. Three groups were set up according to the loaded cells: I, SCs, and IECs (coculture for 3 days) plus IOBs; II, IECs (culture for 3 days) plus IOBs; III, IOBs. Nestin and TIMP-2 expression and osteogenesis of TEBGs were evaluated at 12 weeks post-implantation through histological and radiological assessments.
Results: We found that SC-CM promoted IEC proliferation, migration, capillary-like tube formation, and angiogenesis, but no similar effects were observed for AECs. IECs expressed nestin extensively, while AECs barely expressed nestin, and SC-CM promoted the VEGF secretion of IECs. In the coculture model, SCs promoted nestin and VEGF receptor expression in IECs, and IECs inhibited TIMP-2 expression in SCs. The promotion of prevascularized TEBGs by SCs and IECs in group I augmented new bone formation at 6 and 12 weeks. Nestin expression was higher in group I than in the other groups, while TIMP-2 expression was lower at 12 weeks.
Conclusions: This study demonstrated that SCs can promote TEBG osteogenesis via IECs and further revealed the related specific characteristics of IECs, providing preliminary cytological evidence for neurotization of TEBGs.
Keywords: Bone marrow-derived mesenchymal stem cells; Bone tissue engineering; Endothelial cells; Nestin; Prevascularization; Schwann cells; TIMP-2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells.Biomaterials. 2010 Dec;31(36):9452-61. doi: 10.1016/j.biomaterials.2010.08.036. Epub 2010 Sep 24. Biomaterials. 2010. PMID: 20869769
-
Induced endothelial cells enhance osteogenesis and vascularization of mesenchymal stem cells.Cells Tissues Organs. 2009;190(4):185-93. doi: 10.1159/000218139. Epub 2009 May 6. Cells Tissues Organs. 2009. Retraction in: Cells Tissues Organs. 2010;191(5):430. doi: 10.1159/000313340. Retraction in: Cells Tissues Organs. 2009;190(4):185. doi: 10.1159/000218139. PMID: 19420896 Retracted.
-
Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats.Stem Cell Res Ther. 2017 Jun 5;8(1):134. doi: 10.1186/s13287-017-0592-4. Stem Cell Res Ther. 2017. PMID: 28583167 Free PMC article.
-
Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues.Tissue Eng Part B Rev. 2021 Jun;27(3):199-214. doi: 10.1089/ten.TEB.2020.0132. Epub 2020 Sep 25. Tissue Eng Part B Rev. 2021. PMID: 32854589 Free PMC article. Review.
-
An Up-To-Date Overview of Dental Tissue Regeneration Using Dental Origin Mesenchymal Stem Cells: Challenges and Road Ahead.Front Bioeng Biotechnol. 2022 Apr 12;10:855396. doi: 10.3389/fbioe.2022.855396. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35497335 Free PMC article. Review.
Cited by
-
Neuro-bone tissue engineering: emerging mechanisms, potential strategies, and current challenges.Bone Res. 2023 Dec 20;11(1):65. doi: 10.1038/s41413-023-00302-8. Bone Res. 2023. PMID: 38123549 Free PMC article. Review.
-
Schwann Cells Accelerate Osteogenesis via the Mif/CD74/FOXO1 Signaling Pathway In Vitro.Stem Cells Int. 2022 Jan 13;2022:4363632. doi: 10.1155/2022/4363632. eCollection 2022. Stem Cells Int. 2022. PMID: 35069747 Free PMC article.
-
In vitro dynamic perfusion of prevascularized OECs-DBMs (outgrowth endothelial progenitor cell - demineralized bone matrix) complex fused to recipient vessels in an internal inosculation manner.Bioengineered. 2022 Jun;13(6):14270-14281. doi: 10.1080/21655979.2022.2085560. Bioengineered. 2022. PMID: 35734812 Free PMC article.
-
Sources and applications of endothelial seed cells: a review.Stem Cell Res Ther. 2024 Jun 18;15(1):175. doi: 10.1186/s13287-024-03773-6. Stem Cell Res Ther. 2024. PMID: 38886767 Free PMC article. Review.
-
Bioprinted constructs that simulate nerve-bone crosstalk to improve microenvironment for bone repair.Bioact Mater. 2023 Apr 21;27:377-393. doi: 10.1016/j.bioactmat.2023.02.013. eCollection 2023 Sep. Bioact Mater. 2023. PMID: 37122897 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

