Cybernic treatment with wearable cyborg Hybrid Assistive Limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: a multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001)
- PMID: 34233722
- PMCID: PMC8261928
- DOI: 10.1186/s13023-021-01928-9
Cybernic treatment with wearable cyborg Hybrid Assistive Limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: a multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001)
Abstract
Background: Rare neuromuscular diseases such as spinal muscular atrophy, spinal bulbar muscular atrophy, muscular dystrophy, Charcot-Marie-Tooth disease, distal myopathy, sporadic inclusion body myositis, congenital myopathy, and amyotrophic lateral sclerosis lead to incurable amyotrophy and consequent loss of ambulation. Thus far, no therapeutic approaches have been successful in recovering the ambulatory ability. Thus, the aim of this trial was to evaluate the efficacy and safety of cybernic treatment with a wearable cyborg Hybrid Assistive Limb (HAL, Lower Limb Type) in improving the ambulatory function in those patients.
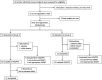
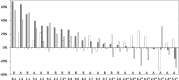
Results: We conducted an open-label, randomised, controlled crossover trial to test HAL at nine hospitals between March 6, 2013 and August 8, 2014. Eligible patients were older than 18 years and had a diagnosis of neuromuscular disease as specified above. They were unable to walk for 10 m independently and had neither respiratory failure nor rapid deterioration in gait. The primary endpoint was the distance passed during a two-minute walk test (2MWT). The secondary endpoints were walking speed, cadence, and step length during the 10-m walk test (10MWT), muscle strength by manual muscle testing (MMT), and a series of functional measures. Adverse events and failures/problems/errors with HAL were also evaluated. Thirty patients were randomly assigned to groups A or B, with each group of 15 receiving both treatments in a crossover design. The efficacy of a 40-min walking program performed nine times was compared between HAL plus a hoist and a hoist only. The final analysis included 13 and 11 patients in groups A and B, respectively. Cybernic treatment with HAL resulted in a 10.066% significantly improved distance in 2MWT (95% confidence interval, 0.667-19.464; p = 0.0369) compared with the hoist only treatment. Among the secondary endpoints, the total scores of MMT and cadence at 10MWT were the only ones that showed significant improvement. The only adverse effects were slight to mild myalgia, back pain, and contact skin troubles, which were easily remedied.
Conclusions: HAL is a new treatment device for walking exercise, proven to be more effective than the conventional method in patients with incurable neuromuscular diseases.
Trial registration: JMACTR, JMA-IIA00156.
Keywords: Cybernics; Gait exercise; Hybrid Assistive Limb (HAL); Neuromuscular disease.
Conflict of interest statement
TN, ST, MN, TSai, KS, AT, TIka, HE, KI, and TIke report grants from MHLW & AMED, non-financial support such as temporary use of the investigation device, HAL-HN01 from CYBERDYNE. Inc., during the conducting of the study. YKo, YA, TSao, CI, TMae, MM, and KK report grants from MHLW, non-financial support such as temporary use of the investigation device, HAL-HN01 from CYBERDYNE. Inc., during the conducting of the study. YI, SM, MA, MI, TMim, TMiu, JM, and YKa report grants from MHLW & AMED during the conducting of the study. HK and YS report grants from MHLW & AMED during the conducting of the study and personal fees from CYBERDYNE. Inc. unrelated to the submitted work. MSh and TH report grants from MHLW & AMED, personal fees from CYBERDYNE, Inc., during the conducting of the study, and personal fees from CYBERDYNE, Inc. unrelated to the submitted work.
Figures


Similar articles
-
The Combined Efficacy of a Two-Year Period of Cybernic Treatment With a Wearable Cyborg Hybrid-Assistive Limb and Leuprorelin Therapy in a Patient With Spinal and Bulbar Muscular Atrophy: A Case Report.Front Neurol. 2022 Jun 24;13:905613. doi: 10.3389/fneur.2022.905613. eCollection 2022. Front Neurol. 2022. PMID: 35812096 Free PMC article.
-
Long-term effects of the gait treatment using a wearable cyborg hybrid assistive limb in a patient with spinal and bulbar muscular atrophy: a case report with 5 years of follow-up.Front Neurol. 2023 Jun 8;14:1143820. doi: 10.3389/fneur.2023.1143820. eCollection 2023. Front Neurol. 2023. PMID: 37360345 Free PMC article.
-
Enhancing the effects of nusinersen with cybernic treatment using Hybrid Assistive Limb (HAL) in spinal muscular atrophy: a real-world case series and exploratory cohort analysis.Orphanet J Rare Dis. 2025 Apr 23;20(1):194. doi: 10.1186/s13023-025-03681-9. Orphanet J Rare Dis. 2025. PMID: 40270050 Free PMC article.
-
Walking and weakness in children: a narrative review of gait and functional ambulation in paediatric neuromuscular disease.J Foot Ankle Res. 2020 Mar 2;13(1):10. doi: 10.1186/s13047-020-0378-2. J Foot Ankle Res. 2020. PMID: 32122377 Free PMC article. Review.
-
Clinical application of the Hybrid Assistive Limb (HAL) for gait training-a systematic review.Front Syst Neurosci. 2015 Mar 25;9:48. doi: 10.3389/fnsys.2015.00048. eCollection 2015. Front Syst Neurosci. 2015. PMID: 25859191 Free PMC article. Review.
Cited by
-
Case Report: Robot-assisted gait training with the wearable cyborg hybrid assistive limb 2S size in three children with cerebral palsy.Front Rehabil Sci. 2025 Mar 24;6:1545105. doi: 10.3389/fresc.2025.1545105. eCollection 2025. Front Rehabil Sci. 2025. PMID: 40196169 Free PMC article.
-
The Effectiveness of Warm-Up Using an Assistive Device in Wheelchair Basketball: A Feasibility Study of Able-Bodied Participants.Cureus. 2024 Sep 5;16(9):e68751. doi: 10.7759/cureus.68751. eCollection 2024 Sep. Cureus. 2024. PMID: 39371762 Free PMC article.
-
Effects of Long-term Hybrid Assistive Limb Use on Gait in Patients with Amyotrophic Lateral Sclerosis.Intern Med. 2022;61(10):1479-1484. doi: 10.2169/internalmedicine.8030-21. Epub 2022 May 15. Intern Med. 2022. PMID: 35569927 Free PMC article.
-
Benefits of a Wearable Cyborg HAL (Hybrid Assistive Limb) in Patients with Childhood-Onset Motor Disabilities: A 1-Year Follow-Up Study.Pediatr Rep. 2023 Mar 9;15(1):215-226. doi: 10.3390/pediatric15010017. Pediatr Rep. 2023. PMID: 36976724 Free PMC article.
-
Feasibility and safety study of wearable cyborg Hybrid Assistive Limb for pediatric patients with cerebral palsy and spinal cord disorders.Front Neurol. 2023 Nov 3;14:1255620. doi: 10.3389/fneur.2023.1255620. eCollection 2023. Front Neurol. 2023. PMID: 38020664 Free PMC article.
References
-
- Sherrington CS. Remarks on some aspects of reflex inhibition; 1925.
-
- Ramón y Cajal S. Cajal's degeneration and regeneration of the nervous system Facsimile of the 1928. London, New York: Hafner Publishing Company; 1991.
-
- Almli CR, Stanley F. Toward a definition of recovery of function. In: Almli CR, Finger S, LeVere TE, Stein D, editors. Brain injury and recovery: theoretical and controversial issues. New York: Plenum; 1988. pp. 1–14.
-
- Bennett RL, Knowlton GC. Overwork weakness in partially denervated skeletal muscle. Clin Orthop. 1958;12:22–29. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

