The site of breast cancer metastases dictates their clonal composition and reversible transcriptomic profile
- PMID: 34233875
- PMCID: PMC8262813
- DOI: 10.1126/sciadv.abf4408
The site of breast cancer metastases dictates their clonal composition and reversible transcriptomic profile
Abstract
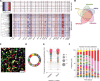
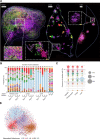
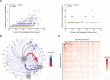
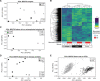
Intratumoral heterogeneity is a driver of breast cancer progression, but the nature of the clonal interactive network involved in this process remains unclear. Here, we optimized the use of optical barcoding to visualize and characterize 31 cancer subclones in vivo. By mapping the clonal composition of thousands of metastases in two clinically relevant sites, the lungs and liver, we found that metastases were highly polyclonal in lungs but not in the liver. Furthermore, the transcriptome of the subclones varied according to their metastatic niche. We also identified a reversible niche-driven signature that was conserved in lung and liver metastases collected during patient autopsies. Among this signature, we found that the tumor necrosis factor-α pathway was up-regulated in lung compared to liver metastases, and inhibition of this pathway affected metastasis diversity. These results highlight that the cellular and molecular heterogeneity observed in metastases is largely dictated by the tumor microenvironment.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Dagogo-Jack I., Shaw A. T., Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

