Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis
- PMID: 34233900
- PMCID: PMC8262447
- DOI: 10.1136/bmj.n1446
Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis
Abstract
Objective: To investigate the efficacy, acceptability, and safety of muscle relaxants for low back pain.
Design: Systematic review and meta-analysis of randomised controlled trials.
Data sources: Medline, Embase, CINAHL, CENTRAL, ClinicalTrials.gov, clinicialtrialsregister.eu, and WHO ICTRP from inception to 23 February 2021.
Eligibility criteria for study selection: Randomised controlled trials of muscle relaxants compared with placebo, usual care, waiting list, or no treatment in adults (≥18 years) reporting non-specific low back pain.
Data extraction and synthesis: Two reviewers independently identified studies, extracted data, and assessed the risk of bias and certainty of the evidence using the Cochrane risk-of-bias tool and Grading of Recommendations, Assessment, Development and Evaluations, respectively. Random effects meta-analytical models through restricted maximum likelihood estimation were used to estimate pooled effects and corresponding 95% confidence intervals. Outcomes included pain intensity (measured on a 0-100 point scale), disability (0-100 point scale), acceptability (discontinuation of the drug for any reason during treatment), and safety (adverse events, serious adverse events, and number of participants who withdrew from the trial because of an adverse event).
Results: 49 trials were included in the review, of which 31, sampling 6505 participants, were quantitatively analysed. For acute low back pain, very low certainty evidence showed that at two weeks or less non-benzodiazepine antispasmodics were associated with a reduction in pain intensity compared with control (mean difference -7.7, 95% confidence interval-12.1 to-3.3) but not a reduction in disability (-3.3, -7.3 to 0.7). Low and very low certainty evidence showed that non-benzodiazepine antispasmodics might increase the risk of an adverse event (relative risk 1.6, 1.2 to 2.0) and might have little to no effect on acceptability (0.8, 0.6 to 1.1) compared with control for acute low back pain, respectively. The number of trials investigating other muscle relaxants and different durations of low back pain were small and the certainty of evidence was reduced because most trials were at high risk of bias.
Conclusions: Considerable uncertainty exists about the clinical efficacy and safety of muscle relaxants. Very low and low certainty evidence shows that non-benzodiazepine antispasmodics might provide small but not clinically important reductions in pain intensity at or before two weeks and might increase the risk of an adverse event in acute low back pain, respectively. Large, high quality, placebo controlled trials are urgently needed to resolve uncertainty.
Systematic review registration: PROSPERO CRD42019126820 and Open Science Framework https://osf.io/mu2f5/.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
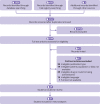
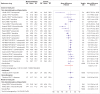
References
-
- James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
