Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab
- PMID: 34233964
- PMCID: PMC8264884
- DOI: 10.1136/jitc-2021-002742
Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab
Abstract
Introduction: Immune checkpoint inhibitors (CPIs) have changed the treatment landscape for many cancers, but also cause severe inflammatory side effects including enterocolitis. CPI-induced enterocolitis is treated empirically with corticosteroids, and infliximab (IFX) is used in corticosteroid-refractory cases. However, robust outcome data for these patients are scarce.
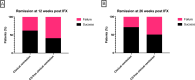
Methods: We conducted a multicenter (six cancer centers), cohort study of outcomes in patients treated with IFX for corticosteroid-refractory CPI-induced enterocolitis between 2007 and 2020. The primary outcome was corticosteroid-free clinical remission (CFCR) with Common Terminology Criteria for Adverse Events (CTCAE) grade 0 for diarrhea at 12 weeks after IFX initiation. We also assessed cancer outcomes at 1 year using RECIST V1.1 criteria.
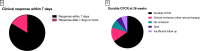
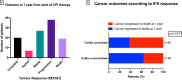
Results: 127 patients (73 male; median age 59 years) were treated with IFX for corticosteroid-refractory CPI-induced enterocolitis. Ninety-six (75.6%) patients had diarrhea CTCAE grade >2 and 115 (90.6%) required hospitalization for colitis. CFCR was 41.2% at 12 weeks and 50.9% at 26 weeks. In multivariable logistic regression, IFX-resistant enterocolitis was associated with rectal bleeding (OR 0.19; 95% CI 0.04 to 0.80; p=0.03) and absence of colonic crypt abscesses (OR 2.16; 95% CI 1.13 to 8.05; p=0.03). Cancer non-progression was significantly more common in patients with IFX-resistant enterocolitis (64.4%) as compared with patients with IFX-responsive enterocolitis (37.5%; p=0.013).
Conclusion: This is the largest study to date reporting outcomes of IFX therapy in patients with corticosteroid-refractory CPI-induced enterocolitis. Using predefined robust endpoints, we have demonstrated that fewer than half of patients achieved CFCR. Our data also indicate that cancer outcomes may be better in patients developing prolonged and severe inflammatory side effects of CPI therapy.
Keywords: autoimmunity; immunotherapy; inflammation.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JLA reports meeting travel support from Vifor Pharma. NP reports he has served as a speaker for Allergan, Bristol Myers Squibb, Falk, Ferring, Janssen, Pfizer, Tillotts, and Takeda, and as a consultant and/or an advisory board member for AbbVie, Allergan, Celgene, Bristol Myers Squibb, Ferring, and Vifor Pharma. DJP received lecture fees from ViiV Healthcare, EISAI, BMS, Roche, Bayer Healthcare and travel expenses from BMS, MSD and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, AstraZeneca, DaVolterra; received research funding (to institution) from MSD, BMS, and Biognosys. SMC reports travel grant from Tesaro and honoraria for educational events from BMS and GSK. JL reports institution grants from Achilles therapeutics, BMS, Merck Sorono, Nektar, Novartis, Pfizer, Roche, Immunocore, Aveo and Pharmacyclics; consulting fees from Iovance, Boston Biomedical, Pfizer, BMS, GSK, Novartis, Incyte, Immunocore, YKT Global, iOnctura and Apple Tree; honoraria from Roche, Novartis, iOnctura, BMS, Pfizer, Incyte, Dynavax, CRUK, GSK, Eisai, Merck, TouchIME and Touch Experts and support for meeting attendance and/or travel from BMS, iOnctura, Roche, Pfizer, Incyte, Merck, Novartis, Pierre Fabre, BUG, ESMO, AIM, AstraZeneca, NCRI, Syneos Health, EUSA, KCA, Bioevents, MedConcept, GSK and RVMais. ST reports grants or contracts from Cancer Research UK (grant reference number C50947/A18176), The National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research (grant reference number A109), The Kidney and Melanoma Cancer Fund of the Royal Marsden Cancer Charity, The Rosetrees Trust (grant reference number A2204) and Ventana Medical Systems Inc (grant reference numbers 10467 and 10530), honoraria from Jules Bordet Institute, Erasmus, Open Health and MD Anderson, support for attending meetings and/or travel from Jules Bordet Institute, ESMO, SMR, Broad, KCA, IFOM, EORTC, ASCO, Ventana, Roche, Institute of Molecular Medicine, KTH Sweden, Pfizer, Erasmus, Systems biology, MD Anderson, WK Weiser, AACR, Research degrees Team, Melanoma Focus and SITC, patents Indel mutations as a therapeutic target and predictive biomarker PCTGB2018/051892 and PCTGB2018/051893 and Clear Cell Renal Cell Carcinoma Biomarkers P113326GB. SP reports honoraria from BMS, MSD, Novartis, Amgen, Gritstone, Zelluna, Achilles, Enara Bio and GSK and travel support from BMS and MSD.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
