Sensitive detection of tumor mutations from blood and its application to immunotherapy prognosis
- PMID: 34234141
- PMCID: PMC8263778
- DOI: 10.1038/s41467-021-24457-2
Sensitive detection of tumor mutations from blood and its application to immunotherapy prognosis
Abstract
Cell-free DNA (cfDNA) is attractive for many applications, including detecting cancer, identifying the tissue of origin, and monitoring. A fundamental task underlying these applications is SNV calling from cfDNA, which is hindered by the very low tumor content. Thus sensitive and accurate detection of low-frequency mutations (<5%) remains challenging for existing SNV callers. Here we present cfSNV, a method incorporating multi-layer error suppression and hierarchical mutation calling, to address this challenge. Furthermore, by leveraging cfDNA's comprehensive coverage of tumor clonal landscape, cfSNV can profile mutations in subclones. In both simulated and real patient data, cfSNV outperforms existing tools in sensitivity while maintaining high precision. cfSNV enhances the clinical utilities of cfDNA by improving mutation detection performance in medium-depth sequencing data, therefore making Whole-Exome Sequencing a viable option. As an example, we demonstrate that the tumor mutation profile from cfDNA WES data can provide an effective biomarker to predict immunotherapy outcomes.
Conflict of interest statement
X.J.Z., W.L., and W.H.W. are co-founders of EarlyDiagnostics Inc. X.N., S.L., and M.L.S. are employees of EarlyDiagnostics Inc. The authors have filed a patent application on methods described in this manuscript. The other authors have no competing interests to declare.
Figures


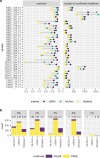
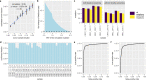
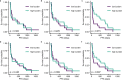
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

