Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis
- PMID: 34234229
- PMCID: PMC8263554
- DOI: 10.1038/s41598-021-93428-w
Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis
Abstract
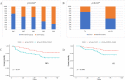
Mounting evidence suggests that microbiota dysbiosis caused by antibiotic administration is a risk factor for cancer, but few research reports focus on the relationships between antibiotics and chemotherapy efficiency. We evaluated the influence of antibiotic administration on neoadjuvant therapy efficacy in patients with breast cancer (BC) in the present study. BC patients were stratified into two groups: antibiotic-treated and control based on antibiotic administration within 30 days after neoadjuvant therapy initiation. Disease-free survival (DFS) and overall survival (OS) were assessed using the Kaplan-Meier method, and the Cox proportional hazards model was used for multivariate analyses. The pathologic complete response rate of the control group was significantly higher than that of the antibiotic-treated group (29.09% vs. 10.20%, p = 0.017). Further univariate analysis with Kaplan-Meier calculations demonstrated that antibiotic administration was strongly linked with both reduced DFS (p = 0.04) at significant statistical levels and OS (p = 0.088) at borderline statistical levels. Antibiotic administration was identified as a significant independent prognostic factor for DFS [hazard ratio (HR) 3.026, 95%, confidence interval (CI) 1.314-6.969, p = 0.009] and OS (HR 2.836, 95% CI 1.016-7.858, p = 0.047) by Cox proportional hazards model analysis. Antibiotics that initiated reduced efficiency of chemotherapy were more noticeable in the HER2-positive subgroup for both DFS (HR 5.51, 95% CI 1.77-17.2, p = 0.003) and OS (HR 7.0395% CI 1.94-25.53, p = 0.003), as well as in the T3-4 subgroup for both DFS (HR 20.36, 95% CI 2.41-172.07, p = 0.006) and OS (HR 13.45, 95% CI 1.39-130.08, p = 0.025) by stratified analysis. Antibiotic administration might be associated with reduced efficacy of neoadjuvant therapy and poor prognosis in BC patients. As a preliminary study, our research made preparations for further understanding and large-scale analyses of the impact of antibiotics on the efficacy of neoadjuvant therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- GLOBOCAN 2020 Graph production, https://gco.iarc.fr/today/home. (2020).
-
- Cortazar P, Kluetz PG. Neoadjuvant breast cancer therapy and drug development. Clin. Adv. Hematol. Oncol. 2015;13:755–761. - PubMed
-
- Guerrero-Zotano AL, Arteaga CL. Neoadjuvant trials in ER+ breast cancer: A tool for acceleration of drug development and discovery. Cancer Discov. 2017;7:561–574. doi: 10.1158/2159-8290.Cd-17-0228. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

