BeEAM conditioning regimen is a safe, efficacious and economical alternative to BEAM chemotherapy
- PMID: 34234243
- PMCID: PMC8263771
- DOI: 10.1038/s41598-021-93516-x
BeEAM conditioning regimen is a safe, efficacious and economical alternative to BEAM chemotherapy
Abstract
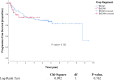
In many stem cell transplant centres, BCNU, etoposide, cytarabine and melphalan (BEAM) high-dose chemotherapy (HDCT) has been replaced by the more economic and available bendamustine, etoposide, cytarabine, melphalan (BeEAM) regimen. However, there is a paucity of information on the efficacy and safety of BeEAM HDCT. We describe our experience with BeEAM HDCT in terms of safety, efficacy and cost-savings. We compare overall and progression-free survival to a cohort of patients previously transplanted at our institution with the older BEAM regimen. We performed a retrospective chart review of 41 lymphoma patients undergoing BeEAM HDCT at the Royal University Hospital in Saskatoon, Saskatchewan between 2015 and 2019 to elicit regimen safety in the first 100 days post-transplant. Furthermore, we calculated overall and progression-free survival and constructed corresponding Kaplan-Meier curves, comparing the results to a historical cohort of BEAM patients (n = 86). Finally, we conducted an economic analysis using the financials available at our centre's pharmacy. With regards to BeEAM HDCT, we report a 100-day transplant-related mortality of 2.4%. Additionally, we report acceptable rates of typhlitis (27%), grade III-IV mucositis (4.9%) and grade III-IV nephrotoxicity (2.4%). In terms of overall and progression-free survival, we found no statistical difference between BeEAM and BEAM (p = 0.296; 0.762, respectively). Finally, our economic analysis revealed a net savings of $21,200 CAD per transplant when BeEAM is used in replacement of BEAM. The acceptable safety profile of BeEAM and its comparable efficacy to BEAM are encouraging for the perseverance of this cost-effective HDCT regimen.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Van Der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N. Engl. J. Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. - DOI - PubMed
-
- Linch D, Goldstone AH, McMillan A, Chopra R, Hudson GV, Winfield D, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: Results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. doi: 10.1016/0140-6736(93)92411-l. - DOI - PubMed
-
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: A randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. - DOI - PubMed
-
- Colita A, Colita A, Bumbea H, Croitoru A, Orban C, Lipan LE, et al. LEAM vs. BEAM vs. CLV conditioning regimen for autologous stem cell transplantation in malignant lymphomas. Retrospective comparison of toxicity and efficacy on 222 patients in the first 100 days after transplant, on behalf of the Romanian society for bone marrow transplantation. Front. Oncol. 2019;9:892. doi: 10.3389/fonc.2019.00892. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

