Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents
- PMID: 34234291
- PMCID: PMC9232615
- DOI: 10.1038/s41433-021-01672-1
Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents
Abstract
Purpose: To evaluate retinal thickness fluctuations in patients with diabetic macular oedema (DMO) treated with anti-vascular endothelial growth factor (anti-VEGF) injections.
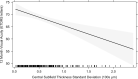
Methods: Visual acuity (VA) and central subfield thickness (CST) were collected at baseline, 3, 6, 9 and 12 months. Retinal thickness fluctuation was quantified by standard deviation (SD) of CST across 12 months. A mixed effects regression model evaluated the relationship between CST SD and VA at 12 months. Multiple linear regression analysis was performed to investigate predictors of CST SD.
Results: Mean baseline and 12-month VAs were 63.5 ± 15.7 and 69.0 ± 13.8 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters (change = +5.1 ± 16.1 letters, p < 0.001). Mean baseline and 12-month CSTs were 396.9 ± 109.7 and 337.7 ± 100.7 μm (change = -59.2 ± 114.8 μm, p < 0.001). Retinal thickness variability across the first 12 months was 59.4 ± 43.6 μm. Stratification of patient eyes by CST SD demonstrated 9.7 letters difference in 12-month VA between first and fourth quartiles. Significant predictors of CST SD include baseline CST, injection type, laser treatment, and DR stage.
Conclusions: Larger retinal thickness fluctuations are associated with poorer visual outcomes in eyes with DMO treated with anti-VEGF injections. Retinal thickness variability may be an important prognostic biomarker for DMO patients.
© 2021. The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
RPS: Genentech/Roche (personal fees), Alcon/Novartis (personal fees), Apellis (grant), Graybug (grant), Zeiss (personal fees), Bausch + Lomb (personal fees), Regeneron Pharmaceuticals, Inc. (personal fees). The other authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

