Sexual arousal gates visual processing during Drosophila courtship
- PMID: 34234348
- PMCID: PMC8973426
- DOI: 10.1038/s41586-021-03714-w
Sexual arousal gates visual processing during Drosophila courtship
Abstract
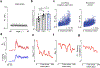
Long-lasting internal arousal states motivate and pattern ongoing behaviour, enabling the temporary emergence of innate behavioural programs that serve the needs of an animal, such as fighting, feeding, and mating. However, how internal states shape sensory processing or behaviour remains unclear. In Drosophila, male flies perform a lengthy and elaborate courtship ritual that is triggered by the activation of sexually dimorphic P1 neurons1-5, during which they faithfully follow and sing to a female6,7. Here, by recording from males as they court a virtual 'female', we gain insight into how the salience of visual cues is transformed by a male's internal arousal state to give rise to persistent courtship pursuit. The gain of LC10a visual projection neurons is selectively increased during courtship, enhancing their sensitivity to moving targets. A concise network model indicates that visual signalling through the LC10a circuit, once amplified by P1-mediated arousal, almost fully specifies a male's tracking of a female. Furthermore, P1 neuron activity correlates with ongoing fluctuations in the intensity of a male's pursuit to continuously tune the gain of the LC10a pathway. Together, these results reveal how a male's internal state can dynamically modulate the propagation of visual signals through a high-fidelity visuomotor circuit to guide his moment-to-moment performance of courtship.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures
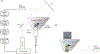

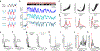



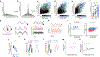








References
-
- Kimura K, Hachiya T, Koganezawa M, Tazawa T. & Yamamoto D. Fruitless and Doublesex Coordinate to Generate Male-Specific Neurons that Can Initiate Courtship. Neuron 59, 759–769 (2008). - PubMed
-
- von Philipsborn AC et al. Neuronal Control of Drosophila Courtship Song. Neuron 69, 509–522 (2011). - PubMed
-
- Yamamoto D. & Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nature Reviews Neuroscience 14, 681–692 (2013). - PubMed
-
- Yu JY, Kanai MI, Demir E, Jefferis GSXE & Dickson BJ Cellular Organization of the Neural Circuit that Drives Drosophila Courtship Behavior. Current Biology 20, 1602–1614 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

