Adverse Events Induced by PD-1/PD-L1 Inhibitors: A Real-World Single-Centre Experience with a Management-Based Approach
- PMID: 34234443
- PMCID: PMC8256379
- DOI: 10.2147/TCRM.S308194
Adverse Events Induced by PD-1/PD-L1 Inhibitors: A Real-World Single-Centre Experience with a Management-Based Approach
Abstract
Aim: To assess the efficacy and tolerance of programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) inhibitors and the impact of a standardised management-based protocol in a real-world setting.
Patients and methods: Data from patients who had received anti-PD-(L)1 were collected from our pharmacy database. Clinical response and toxicity were assessed using RECIST criteria and CTCAE version 5.0, respectively. Overall survival (OS) and progression-free survival (PFS) were estimated with the Kaplan-Meier method. Potential prognostic factors were identified using Cox's model.
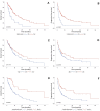
Results: A total of 196 patients and 201 lines of treatment were included (median age: 66 (range: 38-89) years). Types of cancer included non-small cell lung cancer (73%), transitional cell carcinoma (10%), renal cell carcinoma (6%), small cell lung cancer (5%), head and neck squamous cell carcinoma (4%) and classical Hodgkin's lymphoma (1%). Twenty-five (12%) patients had pre-existing autoimmune conditions. Our standardised management-based protocol included 129 (64%) patients. Objective response rate was 29%, median OS was 10 months (IQR: 7-15) and median PFS was 5 months (IQR: 1-22). Patients with an abnormal baseline complete blood count had a worse OS (HR=2.48 [95% CI: 1.24-4.96]; p=0.0103). Thirty-three (16%) patients experienced severe (grade 3 or 4) immune-related adverse event (irAE). There were three (1%) irAE-related deaths. AEs resolved faster when patients were assessed by an internist before anti-PD-(L)1 initiation (p=0.0205).
Conclusion: PD-1 and PD-L1 inhibitors are effective and safe in a real-world setting. Implementation of a standardised management-based protocol with internal medicine specialists is an effective way to optimise irAE management.
Keywords: PD-1 inhibitor; PD-L1 inhibitor; PDL-1 inhibitor; elderly; immune-related adverse events; immunotherapy; prognostic biomarkers; safety; solid tumours.
© 2021 Grimaud et al.
Conflict of interest statement
The authors reported no conflicts of interest for this work.
Figures
Similar articles
-
Comparison of efficacy and safety between PD-1 inhibitors and PD-L1 inhibitors plus platinum-etoposide as first-line treatment for extensive-stage small-cell lung cancer: a multicenter, real-world analysis.BMC Cancer. 2023 Dec 6;23(1):1196. doi: 10.1186/s12885-023-11709-1. BMC Cancer. 2023. PMID: 38057736 Free PMC article.
-
[Real-world study on the efficacy and prognostic predictive biomarker of patients with metastatic non-small cell lung cancer treated with programmed death-1/programmed death ligand 1 inhibitors].Zhonghua Zhong Liu Za Zhi. 2022 May 23;44(5):416-424. doi: 10.3760/cma.j.cn112152-20210709-00504. Zhonghua Zhong Liu Za Zhi. 2022. PMID: 35615798 Chinese.
-
Initial analysis of the synergy of programmed cell death-1 (PD-1) inhibitor and concurrent chemoradiotherapy treatment for recurrent/metastatic head and neck squamous cell carcinoma patients.Radiat Oncol. 2023 Jul 4;18(1):109. doi: 10.1186/s13014-023-02310-8. Radiat Oncol. 2023. PMID: 37403098 Free PMC article.
-
Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis.Cancer Med. 2021 Feb;10(4):1222-1239. doi: 10.1002/cam4.3718. Epub 2021 Jan 19. Cancer Med. 2021. PMID: 33465302 Free PMC article.
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
Cited by
-
Expert consensus on perioperative treatment for non-small cell lung cancer.Transl Lung Cancer Res. 2022 Jul;11(7):1247-1267. doi: 10.21037/tlcr-22-527. Transl Lung Cancer Res. 2022. PMID: 35958323 Free PMC article. Review. No abstract available.
-
Anti-programmed death-1 inhibitor nivolumab-induced immune-related adverse events: hepatitis, renal insufficiency, myositis, vitiligo, and hypothyroidism: a case-based review.Rheumatol Int. 2023 Mar;43(3):559-565. doi: 10.1007/s00296-022-05247-5. Epub 2022 Nov 30. Rheumatol Int. 2023. PMID: 36449057 Review.
-
The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation.Acta Pharm Sin B. 2022 Mar;12(3):1041-1053. doi: 10.1016/j.apsb.2021.09.010. Epub 2021 Sep 16. Acta Pharm Sin B. 2022. PMID: 35530130 Free PMC article. Review.
-
Dendritic Cell-Related Immune Marker CD1C for Predicting Prognosis and Immunotherapy Opportunities of Lung Adenocarcinoma Patients.Appl Biochem Biotechnol. 2024 Dec;196(12):8724-8740. doi: 10.1007/s12010-024-04973-9. Epub 2024 Jun 22. Appl Biochem Biotechnol. 2024. PMID: 38907868
-
Immunotherapy-Related Adverse Events and Clinical Outcomes in Adult Solid-Tumor Patients Admitted to an Onco-Hospitalist Medicine Service.Cancers (Basel). 2025 Jan 25;17(3):403. doi: 10.3390/cancers17030403. Cancers (Basel). 2025. PMID: 39941771 Free PMC article.
References
-
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials