PD-L1 Expressing Recurrent Clear Cell Carcinoma of the Vulva with Durable Partial Response to Pembrolizumab: A Case Report
- PMID: 34234460
- PMCID: PMC8254584
- DOI: 10.2147/OTT.S309661
PD-L1 Expressing Recurrent Clear Cell Carcinoma of the Vulva with Durable Partial Response to Pembrolizumab: A Case Report
Abstract
Background: The optimal treatment and molecular landscape of recurrent clear cell carcinoma of the vulva (VCCC) are unknown. No reported data exist regarding the efficacy of anti-programmed death 1 (PD-1) immune checkpoint inhibition in VCCC. We report on a patient with chemotherapy-refractory recurrent VCCC, who was found to have high tumor programmed death-ligand 1 (PD-L1) combined positive score (CPS), and subsequently experienced a durable partial response (PR), after treatment with off-label fifth-line pembrolizumab.
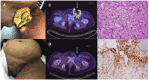
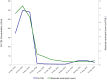
Case presentation: A forty-year-old Filipino woman presented to our center with recurrent VCCC that had progressed on multiple prior lines of cytotoxic chemotherapy. She had a large 25 cm fungating left groin tumor causing marked lower limb lymphedema, pain and limited mobility. PD-L1 CPS by immunohistochemistry was 45. She was treated with off-label pembrolizumab monotherapy and had a dramatic clinical, biochemical and radiological partial response. The progression-free survival of this patient's VCCC after treatment with pembrolizumab, defined as the time from initiation of pembrolizumab until disease progression (by Response Evaluation Criteria in Solid Tumors (version 1.1)), was 8 months. While receiving pembrolizumab, she was diagnosed with concurrent secondary myelodysplastic syndrome with excess blasts (MDS-EB), thought to be related to her prior exposure to multiple lines of cytotoxic chemotherapy. This eventually progressed to acute myeloid leukemia (AML), leading to her demise. Overall survival from time of initiation of pembrolizumab till death was 16 months.
Conclusion: Pembrolizumab was active in this patient with chemotherapy-refractory VCCC which harbored high PD-L1 CPS. Further studies to determine the role of immune check-point blockade in the treatment of VCCC are warranted.
Keywords: clear cell carcinoma; immune check-point blockade; immunotherapy; vulvar cancer.
© 2021 Sachdeva et al.
Conflict of interest statement
Dr David SP Tan reports grants from National Medical Research Council, Singapore, during the conduct of the study; personal fees from Merck Sharp Dohme (MSD), grants from Astra Zeneca, Bayer, Karyopharm Therapeutics, Merck Serono, and Roche, outside the submitted work. All authors declare no other competing interests.
Figures
References
-
- Bolis GB, Maccio T. Clear cell adenocarcinoma of the vulva arising in endometriosis. A case report. Eur J Gynaecol Oncol. 2000;21(4):416–417. - PubMed
-
- Herghelegiu CG, Neacşu A, Oprescu ND, et al. Difficulties of clinical and histopathological diagnosis in advanced vulvar clear cell carcinoma. Rom J Morphol Embryol. 2018;59(4):1233–1237. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

