The economic potential of metalliferous sub-volcanic brines
- PMID: 34234951
- PMCID: PMC8242841
- DOI: 10.1098/rsos.202192
The economic potential of metalliferous sub-volcanic brines
Abstract
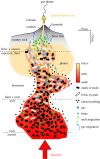
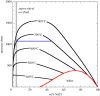
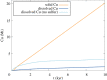
The transition to a low-carbon economy will increase demand for a wide range of metals, notably copper, which is used extensively in power generation and in electric vehicles. Increased demand will require new, sustainable approaches to copper exploration and extraction. Conventional copper mining entails energy-intensive extraction of relatively low-grade ore from large open pits or underground mines and subsequent ore refining. Most copper derives ultimately from hot, hydrous magmatic fluids. Ore formation involves phase separation of these fluids to form copper-rich hypersaline liquids (or 'brines') and subsequent precipitation of copper sulfides. Geophysical surveys of many volcanoes reveal electrically conductive bodies at around 2 km depth, consistent with lenses of brine hosted in porous rock. Building upon emerging concepts in crustal magmatism, we explore the potential of sub-volcanic brines as an in situ source of copper and other metals. Using hydrodynamic simulations, we show that 10 000 years of magma degassing can generate a Cu-rich brine lens containing up to 1.4 Mt Cu in a rock volume of a few km3 at approximately 2 km depth. Direct extraction of metal-rich brines represents a novel development in metal resource extraction that obviates the need for conventional mines, and generates geothermal power as a by-product.
Keywords: copper mining; geothermal energy; ore deposits; volcanoes.
© 2021 The Authors.
Figures





References
-
- Schipper BW, Lin HC, Meloni MA, Wansleeben K, Heijungs R, van der Voet E. 2018. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 132, 28-36. (10.1016/j.resconrec.2018.01.004) - DOI
-
- Valenta RK, Kemp D, Owen JR, Corder GD, Lèbre É. 2019. Re-thinking complex orebodies: consequences for the future world supply of copper. J. Cleaner Prod. 220, 816-826. (10.1016/j.jclepro.2019.02.146) - DOI
-
- Grandell L, Lehtilä A, Kivinen M, Koljonen T, Kihlman S, Lauri LS. 2016. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 95, 53-62. (10.1016/j.renene.2016.03.102) - DOI
-
- Edmonds M, Mather TA, Liu EJ. 2018. A distinct metal fingerprint in arc volcanic emissions. Nat. Geosci. 11, 790. (10.1038/s41561-018-0214-5) - DOI
-
- Hedenquist JW, Lowenstern JB. 1994. The role of magmas in the formation of hydrothermal ore deposits. Nature 370, 519-527. (10.1038/370519a0) - DOI
Associated data
LinkOut - more resources
Full Text Sources

