Autoimmunity as an Etiological Factor of Cancer: The Transformative Potential of Chronic Type 2 Inflammation
- PMID: 34235145
- PMCID: PMC8255631
- DOI: 10.3389/fcell.2021.664305
Autoimmunity as an Etiological Factor of Cancer: The Transformative Potential of Chronic Type 2 Inflammation
Abstract
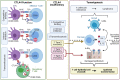
Recent epidemiological studies have found an alarming trend of increased cancer incidence in adults younger than 50 years of age and projected a substantial rise in cancer incidence over the next 10 years in this age group. This trend was exemplified in the incidence of non-cardia gastric cancer and its disproportionate impact on non-Hispanic white females under the age of 50. The trend is concurrent with the increasing incidence of autoimmune diseases in industrialized countries, suggesting a causal link between the two. While autoimmunity has been suspected to be a risk factor for some cancers, the exact mechanisms underlying the connection between autoimmunity and cancer remain unclear and are often controversial. The link has been attributed to several mediators such as immune suppression, infection, diet, environment, or, perhaps most plausibly, chronic inflammation because of its well-recognized role in tumorigenesis. In that regard, autoimmune conditions are common causes of chronic inflammation and may trigger repetitive cycles of antigen-specific cell damage, tissue regeneration, and wound healing. Illustrating the connection between autoimmune diseases and cancer are patients who have an increased risk of cancer development associated with genetically predisposed insufficiency of cytotoxic T lymphocyte-associated protein 4 (CTLA4), a prototypical immune checkpoint against autoimmunity and one of the main targets of cancer immune therapy. The tumorigenic process triggered by CTLA4 insufficiency has been shown in a mouse model to be dependent on the type 2 cytokines interleukin-4 (IL4) and interleukin-13 (IL13). In this type 2 inflammatory milieu, crosstalk with type 2 immune cells may initiate epigenetic reprogramming of epithelial cells, leading to a metaplastic differentiation and eventually malignant transformation even in the absence of classical oncogenic mutations. Those findings complement a large body of evidence for type 1, type 3, or other inflammatory mediators in inflammatory tumorigenesis. This review addresses the potential of autoimmunity as a causal factor for tumorigenesis, the underlying inflammatory mechanisms that may vary depending on host-environment variations, and implications to cancer prevention and immunotherapy.
Keywords: autoimmunity; cancer; chronic inflammation; interleukin-13; interleukin-4; metaplasia; tumorigenesis; type 2 immunity.
Copyright © 2021 Li and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alkhayyat M., Abureesh M., Gill A., Khoudari G., Abou Saleh M., Mansoor E., et al. (2020). Lower rates of colorectal cancer in patients with inflammatory bowel disease using anti-TNF therapy. Inflamm. Bowel Dis. 14:izaa252. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

