C-tactile touch perception in patients with chronic pain disorders
- PMID: 34235344
- PMCID: PMC8253581
- DOI: 10.1097/PR9.0000000000000941
C-tactile touch perception in patients with chronic pain disorders
Abstract
Introduction: Slow brushing over the skin activates C-tactile nerve fibers that transmit pleasant tactile experiences in healthy subjects, leading to an inverted U-shaped velocity dependence of ratings: C-tactile optimal stroking stimulations are rated as more pleasant than slower or faster stimulations. Chronic pain diseases such as postherpetic neuralgia (PHN) and complex regional pain syndrome show altered C-fiber innervation density, sensory loss, and pain sensitization.
Objectives: We aimed to investigate whether C-tactile function is affected in painful conditions.
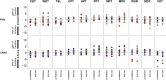
Methods: We assessed psychophysically C-tactile function and sensory perception thresholds in 16 patients with PHN, 19 patients with complex regional pain syndrome, and 22 healthy controls.
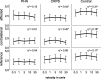
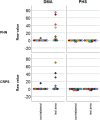
Results: Assessment of C-tactile function showed a significantly altered perceived pleasantness of CT stimulation between healthy controls and patients with chronic pain. In specific, tactile stimulation was perceived less pleasant on the affected and contralateral side when compared with controls. In patients with PHN, velocity-dependent pleasantness ratings could not be obtained, suggesting highly impaired C-tactile function with functional loss of pleasant touch perception.
Conclusions: In conclusion, this is the first report of impaired C-tactile function in patients with PHN. Reduced pleasantness resulting from gentle touch can reflect defective C-fiber function or result from central nervous system effects in a chronic pain state.
Keywords: C-tactile fibers; Neuropathic pain; Pleasant touch; Postherpetic neuralgia.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The International Association for the Study of Pain.
Conflict of interest statement
The authors declare that there is no conflict of interest. Because of regulations of the ethics committee, the full data cannot be made available publicly. However, data access will be provided to other researchers on request.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Mohr Drewes A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. - PubMed
-
- Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain 2000;16(2 suppl):S12–20. - PubMed
-
- Buonocore M, Gatti AM, Amato G, Aloisi AM, Bonezzi C. Allodynic skin in post-herpetic neuralgia: histological correlates. J Cell Physiol 2012;227:934–8. - PubMed
LinkOut - more resources
Full Text Sources
