Changes in blood pressure after crossover to ultrasound renal denervation in patients initially treated with sham in the RADIANCE-HTN SOLO trial
- PMID: 34236037
- PMCID: PMC9724980
- DOI: 10.4244/EIJ-D-21-00295
Changes in blood pressure after crossover to ultrasound renal denervation in patients initially treated with sham in the RADIANCE-HTN SOLO trial
Abstract
Background: The multicentre, randomised, sham-controlled RADIANCE-HTN SOLO trial reported the blood pressure (BP)-lowering efficacy and safety of ultrasound renal denervation (RDN) in the absence (2 months) and presence (6 and 12 months) of antihypertensive medications in patients with mild-to-moderate hypertension.
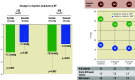
Aims: The aim of this report was to evaluate patients originally assigned to the sham group who crossed over to RDN.
Methods: After the primary endpoint was met, patients in the sham arm who remained uncontrolled were allowed to cross over to receive RDN. All patients were unblinded and treated with standard of care medications at the time of crossover. Ambulatory BP was evaluated 2 and 6 months after crossover.
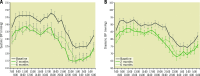
Results: Among 72 subjects of the sham arm, 33 underwent ultrasound RDN after an average follow-up of 23±6 months. Prior to crossover, patients had a daytime ambulatory BP of 144.1±10.1/89.9±8.4 mmHg and received 1.2±0.8 antihypertensive medications. Mean change in daytime ambulatory BP from pre-crossover to 2 and 6 months post RDN was -11.2±13.7/-7.1±8.9 mmHg (n=33; p<0.001; p<0.001) and -10.8±17.3/-7.8±11.6 mmHg (n=27; p=0.002; p<0.001). The number of antihypertensive medications did not change from pre-crossover baseline to 2 and 6 months. Eighteen of 33 (54.5%) patients had their daytime ambulatory BP controlled (<135/85 mmHg) at 2 months and 44.4% (12/27) at 6 months post RDN. No major procedure-related adverse events occurred.
Conclusions: During unblinded long-term follow-up of the RADIANCE-HTN SOLO study, patients originally assigned to a sham procedure who remained uncontrolled had significant reductions in BP following crossover treatment with ultrasound RDN.
Conflict of interest statement
F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), and Deutsche Forschungsgemeinschaft (SFB TRR219) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic and ReCor Medical. M.J. Bloch has received personal fees from ReCor Medical and Medtronic. M. Azizi has received research grants from the French Ministry of Health, Quantum Genomics and the European H2020 program, has received grant support and non-financial support from ReCor Medical and Idorsia, and has received personal fees from CVRx. R.E. Schmieder has received grant support and personal fees from ReCor Medical, Medtronic, and Ablative Solutions. M.D. Lobo has received personal fees from ReCor Medical, Medtronic, CVRx, Ablative Solutions, Vascular Dynamics, ROX Medical and Tarilan Laser Technologies and grants from Medtronic. A.S.P. Sharp has received personal fees from ReCor Medical, Medtronic and Philips. J. Daemen has received grant support from ReCor Medical, Medtronic, Boston Scientific, Abbott Vascular, Acist Medical, AstraZeneca, Pie Medical, and PulseCath, and has received consulting fees from ReCor Medical, Medtronic, Acist Medical, Boston Scientific, Pie Medical, Siemens, and PulseCath. J. Basile has received grant support from ReCor Medical and Ablative Solutions. M.A. Weber has received personal fees from ReCor Medical, Medtronic, Boston Scientific, and Ablative Solutions. A. Scicli is an employee of ReCor Medical. C.K. McClure is an employee of NAMSA, a contractor for ReCor Medical. A.J. Kirtane reports institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, and ReCor Medical. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for speaking engagements and/or consulting. He reports personal fees for consulting from Neurotronic, and travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Y. Wang has no conflicts of interests to declare.
Figures


References
-
- James PA, Ogedegbe O, Narva AS, Wright JT, Townsend RR, Taler SJ, Svetkey LP, Smith SC Jr, MacKenzie TD, Oparil S, LeFevre ML, Lackland DT, Handler J, Dennison-Himmelfarb C, Cushman WC, Carter BL, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. - DOI - PubMed
-
- Benjamin EJ, Sasson C, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Towfighi A, Nasir K, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Neumar RW, Mussolino ME, Blaha MJ, Isasi CR, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Jiménez MC, Mozaffarian D, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Muntner P American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

