SAHA could inhibit TGF-β1/p38 pathway in MI-induced cardiac fibrosis through DUSP4 overexpression
- PMID: 34236463
- PMCID: PMC8732849
- DOI: 10.1007/s00380-021-01900-4
SAHA could inhibit TGF-β1/p38 pathway in MI-induced cardiac fibrosis through DUSP4 overexpression
Abstract
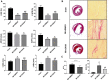
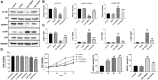
Growing evidences have revealed that a histone deacetylase inhibitor (HDACi), suberoylanilide hydroxamic acid (SAHA) has anti-fibrotic effect in different diseases. In this study, we first evaluated whether SAHA could suppress cardiac fibrosis. Mice with MI-induced cardiac fibrosis were treated with SAHA by intraperitoneal injection and their cardiac function was improved after SAHA treatment. Results of western blotting and qRT-PCR in heart tissues suggested that TGFβ1/P38 pathway was activated in MI mice, and this effect was reversed by SAHA. Cell proliferation assay suggested that SAHA could suppress TGF-β1-induced cardiac fibroblasts proliferation. Furthermore, results of western blotting and qRT-PCR in cardiac fibroblasts depicted that SAHA may exert its anti-fibrotic effect through inhibiting TGF-β1-induced P38 phosphorylation by promoting DUSP4 expression. Our findings may substantiate SAHA as a promising treatment for MI-induced cardiac fibrosis.
Keywords: Cardiac fibrosis; Dual-specificity phosphatase 4 (DUSP4); Suberoylanilide hydroxamic acid (SAHA); TGF-β1.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

