A theoretical approach for the acylation/deacylation mechanisms of avibactam in the reversible inhibition of KPC-2
- PMID: 34236545
- PMCID: PMC8264174
- DOI: 10.1007/s10822-021-00408-3
A theoretical approach for the acylation/deacylation mechanisms of avibactam in the reversible inhibition of KPC-2
Abstract
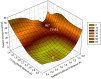
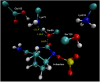
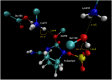
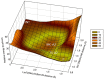
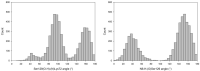
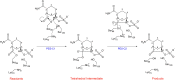
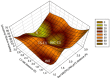
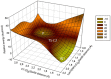
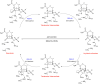
Klebsiella pneumoniae carbapenemase (KPC-2) is the most commonly encountered class A β-lactamase variant worldwide, which confer high-level resistance to most available antibiotics. In this article we address the issue by a combined approach involving molecular dynamics simulations and hybrid quantum mechanics/molecular mechanics calculations. The study contributes to improve the understanding, at molecular level, of the acylation and deacylation stages of avibactam involved in the inhibition of KPC-2. The results show that both mechanisms, acylation and deacylation, the reaction occur via the formation of a tetrahedral intermediate. The formation of this intermediate corresponds to the rate limiting stage. The activation barriers are 19.5 kcal/mol and 23.0 kcal/mol for the acylation and deacylation stages, respectively. The associated rate constants calculated, using the Eyring equation, are 1.2 × 10-1 and 3.9 × 10-4 (s-1). These values allow estimating a value of 3.3 × 10-3 for the inhibition constant, in good agreement with the experimental value.
Keywords: Avibactam; Inhibition; KPC-2; QM/MM.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Figures












References
-
- World Health Organization (2020) Antimicrobial resistance. World Health Organization, Geneva. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
-
- World Health Organization (2020) Lack of new antibiotics threatens global efforts to contain drug-resistant infections. World Health Organization, Geneva. https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threate...
-
- Centers for Disease Control and Prevention (2019) Biggest threats and data. Centers for Disease Control and Prevention, Atlanta. https://www.cdc.gov/drugresistance/biggest-threats.html
-
- Centers for Disease Control and Prevention (2020) About antibiotic resistance. Centers for Disease Control and Prevention, Atlanta. https://www.cdc.gov/drugresistance/about.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

