Physiologic Response to the Pfizer-BioNTech COVID-19 Vaccine Measured Using Wearable Devices: Prospective Observational Study
- PMID: 34236995
- PMCID: PMC8341091
- DOI: 10.2196/28568
Physiologic Response to the Pfizer-BioNTech COVID-19 Vaccine Measured Using Wearable Devices: Prospective Observational Study
Abstract
Background: The Pfizer-BioNTech COVID-19 vaccine uses a novel messenger RNA technology to elicit a protective immune response. Short-term physiologic responses to the vaccine have not been studied using wearable devices.
Objective: We aim to characterize physiologic changes in response to COVID-19 vaccination in a small cohort of participants using a wearable device (WHOOP Strap 3.0). This is a proof of concept for using consumer-grade wearable devices to monitor response to COVID-19 vaccines.
Methods: In this prospective observational study, physiologic data from 19 internal medicine residents at a single institution that received both doses of the Pfizer-BioNTech COVID-19 vaccine was collected using the WHOOP Strap 3.0. The primary outcomes were percent change from baseline in heart rate variability (HRV), resting heart rate (RHR), and respiratory rate (RR). Secondary outcomes were percent change from baseline in total, rapid eye movement, and deep sleep. Exploratory outcomes included local and systemic reactogenicity following each dose and prophylactic analgesic use.
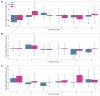
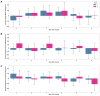
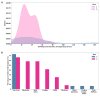
Results: In 19 individuals (mean age 28.8, SD 2.2 years; n=10, 53% female), HRV was decreased on day 1 following administration of the first vaccine dose (mean -13.44%, SD 13.62%) and second vaccine dose (mean -9.25%, SD 22.6%). RHR and RR showed no change from baseline after either vaccine dose. Sleep duration was increased up to 4 days post vaccination, after an initial decrease on day 1. Increased sleep duration prior to vaccination was associated with a greater change in HRV. Local and systemic reactogenicity was more severe after dose two.
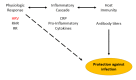
Conclusions: This is the first observational study of the physiologic response to any of the novel COVID-19 vaccines as measured using wearable devices. Using this relatively small healthy cohort, we provide evidence that HRV decreases in response to both vaccine doses, with no significant changes in RHR or RR. Sleep duration initially decreased following each dose with a subsequent increase thereafter. Future studies with a larger sample size and comparison to other inflammatory and immune biomarkers such as antibody response will be needed to determine the true utility of this type of continuous wearable monitoring in regards to vaccine responses. Our data raises the possibility that increased sleep prior to vaccination may impact physiologic responses and may be a modifiable way to increase vaccine response. These results may inform future studies using wearables for monitoring vaccine responses.
Trial registration: ClinicalTrials.gov NCT04304703; https://www.clinicaltrials.gov/ct2/show/NCT04304703.
Keywords: COVID-19; REM sleep; cohort; deep sleep; heart rate; heart rate variability; monitoring; physiological; remote physiologic monitoring; respiratory; respiratory rate; sleep; vaccine; wearable; wearable devices.
©Alexander G Hajduczok, Kara M DiJoseph, Brinnae Bent, Audrey K Thorp, Jon B Mullholand, Stuart A MacKay, Sabrina Barik, Jamie J Coleman, Catharine I Paules, Andrew Tinsley. Originally published in JMIR Formative Research (https://formative.jmir.org), 04.08.2021.
Conflict of interest statement
Conflicts of Interest: CIP is a consultant for Axle Informatics for work not related to this manuscript. CIP is also the site PI for the Adaptive COVID-19 Treatment Trial (ACTT) and the ACTIV-5 / Big Effect Trial (BET-B) for the Treatment of COVID-19 which receive funding from the National Institutes of Health. This work is not related to the submitted manuscript.
Figures

References
-
- COVID-19. Centers for Disease Control and Prevention. [2003-04-21]. https://www.cdc.gov/coronavirus/2019-ncov/index.html)
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Johns Hopkins Coronavirus Resource Center. [2003-04-21]. https://coronavirus.jhu.edu/map.html.
-
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261–279. doi: 10.1038/nrd.2017.243. http://europepmc.org/abstract/MED/29326426 - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. http://europepmc.org/abstract/MED/33301246 - DOI - PMC - PubMed
-
- Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A, Pascal K, Maurus D, Brachtendorf S, Lörks V, Sikorski J, Koch P, Hilker R, Becker D, Eller AK, Grützner J, Tonigold M, Boesler C, Rosenbaum C, Heesen L, Kühnle MC, Poran A, Dong JZ, Luxemburger U, Kemmer-Brück A, Langer D, Bexon M, Bolte S, Palanche T, Schultz A, Baumann S, Mahiny AJ, Boros G, Reinholz J, Szabó GT, Karikó K, Shi PY, Fontes-Garfias C, Perez JL, Cutler M, Cooper D, Kryatsous CA, Dormitzer PR, Jansen KU, Türeci Ö. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. Preprint posted online on December 11, 2020.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

