Functional Profile of Systemic and Intrathecal Cebranopadol in Nonhuman Primates
- PMID: 34237134
- PMCID: PMC8446297
- DOI: 10.1097/ALN.0000000000003848
Functional Profile of Systemic and Intrathecal Cebranopadol in Nonhuman Primates
Abstract
Background: Cebranopadol, a mixed nociceptin/opioid receptor full agonist, can effectively relieve pain in rodents and humans. However, it is unclear to what degree different opioid receptor subtypes contribute to its antinociception and whether cebranopadol lacks acute opioid-associated side effects in primates. The authors hypothesized that coactivation of nociceptin receptors and μ receptors produces analgesia with reduced side effects in nonhuman primates.
Methods: The antinociceptive, reinforcing, respiratory-depressant, and pruritic effects of cebranopadol in adult rhesus monkeys (n = 22) were compared with μ receptor agonists fentanyl and morphine using assays, including acute thermal nociception, IV drug self-administration, telemetric measurement of respiratory function, and itch-scratching responses.
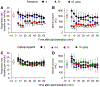
Results: Subcutaneous cebranopadol (ED50, 2.9 [95% CI, 1.8 to 4.6] μg/kg) potently produced antinociception compared to fentanyl (15.8 [14.6 to 17.1] μg/kg). Pretreatment with antagonists selective for nociceptin and μ receptors, but not δ and κ receptor antagonists, caused rightward shifts of the antinociceptive dose-response curve of cebranopadol with dose ratios of 2 and 9, respectively. Cebranopadol produced reinforcing effects comparable to fentanyl, but with decreased reinforcing strength, i.e., cebranopadol (mean ± SD, 7 ± 3 injections) versus fentanyl (12 ± 3 injections) determined by a progressive-ratio schedule of reinforcement. Unlike fentanyl (8 ± 2 breaths/min), systemic cebranopadol at higher doses did not decrease the respiratory rate (17 ± 2 breaths/min). Intrathecal cebranopadol (1 μg) exerted full antinociception with minimal scratching responses (231 ± 137 scratches) in contrast to intrathecal morphine (30 μg; 3,009 ± 1,474 scratches).
Conclusions: In nonhuman primates, the μ receptor mainly contributed to cebranopadol-induced antinociception. Similar to nociceptin/μ receptor partial agonists, cebranopadol displayed reduced side effects, such as a lack of respiratory depression and pruritus. Although cebranopadol showed reduced reinforcing strength, its detectable reinforcing effects and strength warrant caution, which is critical for the development and clinical use of cebranopadol.
Copyright © 2021, the American Society of Anesthesiologists. All Rights Reserved.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

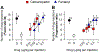

Comment in
-
Does Divergence Exist between Animal and Human Data on the Effect of Cebranopadol?Anesthesiology. 2021 Sep 1;135(3):382-383. doi: 10.1097/ALN.0000000000003885. Anesthesiology. 2021. PMID: 34329373 No abstract available.
Similar articles
-
Opioid-type Respiratory Depressant Side Effects of Cebranopadol in Rats Are Limited by Its Nociceptin/Orphanin FQ Peptide Receptor Agonist Activity.Anesthesiology. 2017 Apr;126(4):708-715. doi: 10.1097/ALN.0000000000001530. Anesthesiology. 2017. PMID: 28291086
-
Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates.J Pharmacol Exp Ther. 2012 Oct;343(1):72-81. doi: 10.1124/jpet.112.194308. Epub 2012 Jun 28. J Pharmacol Exp Ther. 2012. PMID: 22743574 Free PMC article.
-
Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys.J Pain. 2009 May;10(5):509-16. doi: 10.1016/j.jpain.2008.11.006. Epub 2009 Feb 23. J Pain. 2009. PMID: 19231294 Free PMC article.
-
Nociceptin/orphanin FQ receptor ligands and translational challenges: focus on cebranopadol as an innovative analgesic.Br J Anaesth. 2018 Nov;121(5):1105-1114. doi: 10.1016/j.bja.2018.06.024. Epub 2018 Aug 22. Br J Anaesth. 2018. PMID: 30336855 Free PMC article.
-
Cebranopadol : a first-in-class potent analgesic agent with agonistic activity at nociceptin/orphanin FQ and opioid receptors.Expert Opin Investig Drugs. 2015 Jun;24(6):837-44. doi: 10.1517/13543784.2015.1036985. Epub 2015 Apr 12. Expert Opin Investig Drugs. 2015. PMID: 25865744 Review.
Cited by
-
Potential therapeutic targets for the treatment of opioid abuse and pain.Adv Pharmacol. 2022;93:335-371. doi: 10.1016/bs.apha.2021.09.002. Epub 2021 Nov 9. Adv Pharmacol. 2022. PMID: 35341570 Free PMC article. Review.
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders.Mol Brain. 2022 Nov 24;15(1):95. doi: 10.1186/s13041-022-00982-z. Mol Brain. 2022. PMID: 36434658 Free PMC article. Review.
-
Functional roles of neuromedin B and gastrin-releasing peptide in regulating itch and pain in the spinal cord of non-human primates.Biochem Pharmacol. 2022 Apr;198:114972. doi: 10.1016/j.bcp.2022.114972. Epub 2022 Feb 18. Biochem Pharmacol. 2022. PMID: 35189108 Free PMC article.
-
Nociceptin Receptor-Related Agonists as Safe and Non-addictive Analgesics.Drugs. 2023 Jun;83(9):771-793. doi: 10.1007/s40265-023-01878-5. Epub 2023 May 20. Drugs. 2023. PMID: 37209211 Free PMC article. Review.
-
Has the United States Reached a Plateau in Overdoses Caused by Synthetic Opioids After the Onset of the COVID-19 Pandemic? Examination of Centers for Disease Control and Prevention Data to November 2021.Front Psychiatry. 2022 Jul 7;13:947603. doi: 10.3389/fpsyt.2022.947603. eCollection 2022. Front Psychiatry. 2022. PMID: 35873233 Free PMC article.
References
-
- Hewson DW, Struys M, Hardman JG: Opioids: refining the perioperative role of God’s own medicine. Br J Anaesth. 2019; 122:e93–e5 - PubMed
-
- Volkow ND, McLellan AT: Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. The New England journal of medicine. 2016; 374:1253–63 - PubMed
-
- Azzam AAH, McDonald J, Lambert DG: Hot topics in opioid pharmacology: mixed and biased opioids. Br J Anaesth. 2019; 122:e136–e45 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

