Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice
- PMID: 34237282
- PMCID: PMC8387467
- DOI: 10.1016/j.ajhg.2021.06.010
Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice
Abstract
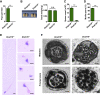
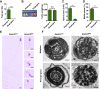
Multiple morphological abnormalities of the sperm flagella (MMAF)-induced asthenoteratozoospermia is a common cause of male infertility. Previous studies have identified several MMAF-associated genes, highlighting the condition's genetic heterogeneity. To further define the genetic causes underlying MMAF, we performed whole-exome sequencing in a cohort of 643 Chinese MMAF-affected men. Bi-allelic DNAH10 variants were identified in five individuals with MMAF from four unrelated families. These variants were either rare or absent in public population genome databases and were predicted to be deleterious by multiple bioinformatics tools. Morphological and ultrastructural analyses of the spermatozoa obtained from men harboring bi-allelic DNAH10 variants revealed striking flagellar defects with the absence of inner dynein arms (IDAs). DNAH10 encodes an axonemal IDA heavy chain component that is predominantly expressed in the testes. Immunostaining analysis indicated that DNAH10 localized to the entire sperm flagellum of control spermatozoa. In contrast, spermatozoa from the men harboring bi-allelic DNAH10 variants exhibited an absence or markedly reduced staining intensity of DNAH10 and other IDA components, including DNAH2 and DNAH6. Furthermore, the phenotypes were recapitulated in mouse models lacking Dnah10 or expressing a disease-associated variant, confirming the involvement of DNAH10 in human MMAF. Altogether, our findings in humans and mice demonstrate that DNAH10 is essential for sperm flagellar assembly and that deleterious bi-allelic DNAH10 variants can cause male infertility with MMAF. These findings will provide guidance for genetic counseling and insights into the diagnosis of MMAF-associated asthenoteratozoospermia.
Keywords: DNAH10; MMAF; knockout mice; male infertility; sperm flagella.
Copyright © 2021 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

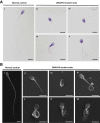
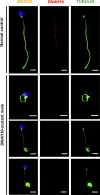
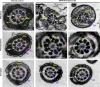
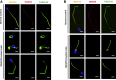
Similar articles
-
Bi-allelic variants in DNAH10 cause asthenoteratozoospermia and male infertility.J Assist Reprod Genet. 2022 Jan;39(1):251-259. doi: 10.1007/s10815-021-02306-x. Epub 2021 Oct 16. J Assist Reprod Genet. 2022. PMID: 34657236 Free PMC article.
-
Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility.Am J Hum Genet. 2020 Aug 6;107(2):330-341. doi: 10.1016/j.ajhg.2020.06.004. Epub 2020 Jul 2. Am J Hum Genet. 2020. PMID: 32619401 Free PMC article.
-
Patients with mutations in DNAH2, DNAH6 and DNAH10 causing multiple morphological abnormalities of human sperm flagella achieve good ICSI outcomes.Reprod Biomed Online. 2025 Aug;51(2):104949. doi: 10.1016/j.rbmo.2025.104949. Epub 2025 Mar 20. Reprod Biomed Online. 2025. PMID: 40592014
-
A novel variant in CFAP69 causes asthenoteratozoospermia with treatable ART outcomes and a literature review.J Assist Reprod Genet. 2023 Sep;40(9):2175-2184. doi: 10.1007/s10815-023-02873-1. Epub 2023 Jul 1. J Assist Reprod Genet. 2023. PMID: 37392306 Free PMC article. Review.
-
Insight on multiple morphological abnormalities of sperm flagella in male infertility: what is new?Asian J Androl. 2020 May-Jun;22(3):236-245. doi: 10.4103/aja.aja_53_19. Asian J Androl. 2020. PMID: 31210147 Free PMC article. Review.
Cited by
-
Novel mutations in DNAH17 cause sperm flagellum defects and their influence on ICSI outcome.J Assist Reprod Genet. 2023 Oct;40(10):2485-2492. doi: 10.1007/s10815-023-02897-7. Epub 2023 Aug 14. J Assist Reprod Genet. 2023. PMID: 37574497 Free PMC article. Review.
-
Mitochondrial regulation during male germ cell development.Cell Mol Life Sci. 2022 Jan 24;79(2):91. doi: 10.1007/s00018-022-04134-3. Cell Mol Life Sci. 2022. PMID: 35072818 Free PMC article. Review.
-
Association of novel DNAH11 variants with asthenoteratozoospermia lead to male infertility.Hum Genomics. 2024 Sep 11;18(1):97. doi: 10.1186/s40246-024-00658-w. Hum Genomics. 2024. PMID: 39256880 Free PMC article.
-
Homozygous mutation in DNALI1 leads to asthenoteratozoospermia by affecting the inner dynein arms.Front Endocrinol (Lausanne). 2023 Jan 16;13:1058651. doi: 10.3389/fendo.2022.1058651. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36726469 Free PMC article.
-
Bi-allelic variants in human WDR63 cause male infertility via abnormal inner dynein arms assembly.Cell Discov. 2021 Nov 16;7(1):110. doi: 10.1038/s41421-021-00327-5. Cell Discov. 2021. PMID: 34782613 Free PMC article.
References
-
- Tournaye H., Krausz C., Oates R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:544–553. - PubMed
-
- Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S., Arafa M., Panner Selvam M.K., Shah R. Male infertility. Lancet. 2021;397:319–333. - PubMed
-
- Shahrokhi S.Z., Salehi P., Alyasin A., Taghiyar S., Deemeh M.R. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia. 2020;52:e13463. - PubMed
-
- Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. - PMC - PubMed
-
- Wang Z., Pan Y., He L., Song X., Chen H., Pan C., Qu L., Zhu H., Lan X. Multiple morphological abnormalities of the sperm flagella (MMAF)-associated genes: The relationships between genetic variation and litter size in goats. Gene. 2020;753:144778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

