Recruiting and retaining parents in behavioral intervention trials: Strategies to consider
- PMID: 34237457
- PMCID: PMC8453094
- DOI: 10.1016/j.cct.2021.106502
Recruiting and retaining parents in behavioral intervention trials: Strategies to consider
Abstract
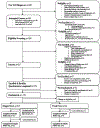
Objective: Recruitment and retention are paramount to the success of randomized controlled trials (RCTs); however, strategies and challenges to optimize recruitment and retention are often omitted from outcomes papers. The current manuscript presents strategies used to recruit and retain over 97% parents of young children newly diagnosed with type 1 diabetes for over 15-months post-randomization enrolled in First STEPS, a behavioral, two-site RCT.
Method: Participants included 157 primary caregivers of young children newly diagnosed with type 1 diabetes. Recruitment and retention strategies are described and include collaboration with medical teams, careful selection and training of study staff, inclusion of a behavioral run-in prior to randomization, financial incentives, creation of a study identity using retention items, obtainment of feedback from community stakeholders, and minimization of participant burden.
Results: Use of recruitment and retention strategies resulted in enrollment of 58% of eligible and reached families, with retention of the enrolled sample above 97% for over 15 months. Participants reported high acceptability of and satisfaction with specific recruitment and retention strategies.
Conclusions: The strategies used to recruit and retain caregivers of young children newly diagnosed with a chronic illness were feasible to implement within multidisciplinary diabetes clinics and may apply to other pediatric populations. Future research may benefit from a focus on strategies to engage more diverse samples.
Trial registration: ClinicalTrials.gov identifier: NCT02527525.
Keywords: Clinical trial; Recruitment; Retention; Type 1 diabetes.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors have indicated that they have no financial relationships relevant or known conflicts of interest to this article to disclose.
Figures
References
-
- Barakat LP, Patterson CA, Mondestin V, Chavez V, Austin T, Robinson MR, Li Y, Smith-Whitley K, & Cohen R (2013). Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemporary Clinical Trials, 34(2), 218–226. 10.1016/j.cct.2012.11.001 - DOI - PubMed
-
- Dunn MJ, Rodriguez EM, Barnwell AS, Grossenbacher JC, Vannatta K, Gerhardt CA, & Compas BE (2012). Posttraumatic stress symptoms in parents of children with cancer within six months of diagnosis. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 31(2), 176–185. 10.1037/a0025545 - DOI - PMC - PubMed
-
- Greenberg RG, Gamel B, Bloom D, Bradley J, Jafri HS, Hinton D, Nambiar S, Wheeler C, Tiernan R, Smith PB, Roberts J, & Benjamin DK Jr (2017). Parents’ perceived obstacles to pediatric clinical trial participation: Findings from the clinical trials transformation initiative. Contemporary Clinical Trials Communications, 9, 33–39. 10.1016/j.conctc.2017.11.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical