Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease
- PMID: 34237462
- PMCID: PMC8551217
- DOI: 10.1016/j.jcmgh.2021.07.001
Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease
Erratum in
-
Corrections.Cell Mol Gastroenterol Hepatol. 2024;17(4):670. doi: 10.1016/j.jcmgh.2024.02.005. Epub 2024 Feb 21. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38383221 Free PMC article. No abstract available.
Abstract
Background & aims: The gastrointestinal epithelium plays a crucial role in maintaining homeostasis with the gut microbiome. Mucins are essential for intestinal barrier function and serve as a scaffold for antimicrobial factors. Mucin 2 (MUC2) is the major intestinal gel-forming mucin produced predominantly by goblet cells. Goblet cells express anterior gradient 2 (AGR2), a protein disulfide isomerase that is crucial for proper processing of gel-forming mucins. Here, we investigated 2 siblings who presented with severe infantile-onset inflammatory bowel disease.
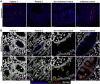
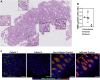
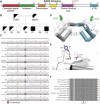
Methods: We performed whole-genome sequencing to identify candidate variants. We quantified goblet cell numbers using H&E histology and investigated the expression of gel-forming mucins, stress markers, and goblet cell markers using immunohistochemistry. AGR2-MUC2 binding was evaluated using co-immunoprecipitation. Endoplasmic reticulum (ER) stress regulatory function of mutant AGR2 was examined by expression studies in Human Embryonic Kidney 293T (HEK293T) using tunicamycin to induce ER stress.
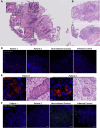
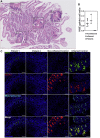
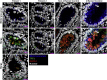
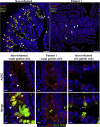
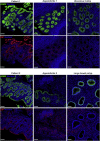
Results: Both affected siblings were homozygous for a missense variant in AGR2. Patient biopsy specimens showed reduced goblet cells; depletion of MUC2, MUC5AC, and MUC6; up-regulation of AGR2; and increased ER stress. The mutant AGR2 showed reduced capacity to bind MUC2 and alleviate tunicamycin-induced ER stress.
Conclusions: Phenotype-genotype segregation, functional experiments, and the striking similarity of the human phenotype to AGR2-/- mouse models suggest that the AGR2 missense variant is pathogenic. The Mendelian deficiency of AGR2, termed "Enteropathy caused by AGR2 deficiency, Goblet cell Loss, and ER Stress" (EAGLES), results in a mucus barrier defect, the inability to mitigate ER stress, and causes infantile-onset inflammatory bowel disease.
Keywords: AGR2; ER Stress; Goblet Cells; Intestinal Metaplasia; MUC2.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis.PLoS Med. 2008 Mar 4;5(3):e54. doi: 10.1371/journal.pmed.0050054. PLoS Med. 2008. PMID: 18318598 Free PMC article.
-
Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice.Dev Biol. 2010 Feb 15;338(2):270-9. doi: 10.1016/j.ydbio.2009.12.008. Epub 2009 Dec 16. Dev Biol. 2010. PMID: 20025862 Free PMC article.
-
IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells.Gastroenterology. 2013 Feb;144(2):357-368.e9. doi: 10.1053/j.gastro.2012.10.043. Epub 2012 Oct 31. Gastroenterology. 2013. PMID: 23123183
-
Intestinal goblet cells and mucins in health and disease: recent insights and progress.Curr Gastroenterol Rep. 2010 Oct;12(5):319-30. doi: 10.1007/s11894-010-0131-2. Curr Gastroenterol Rep. 2010. PMID: 20703838 Free PMC article. Review.
-
Orchestration of MUC2 - The key regulatory target of gut barrier and homeostasis: A review.Int J Biol Macromol. 2023 May 1;236:123862. doi: 10.1016/j.ijbiomac.2023.123862. Epub 2023 Mar 2. Int J Biol Macromol. 2023. PMID: 36870625 Review.
Cited by
-
The role of mesenchymal stem cells in attenuating inflammatory bowel disease through ubiquitination.Front Immunol. 2024 Aug 9;15:1423069. doi: 10.3389/fimmu.2024.1423069. eCollection 2024. Front Immunol. 2024. PMID: 39185411 Free PMC article. Review.
-
The mechanism of traditional medicine in alleviating ulcerative colitis: regulating intestinal barrier function.Front Pharmacol. 2023 Oct 9;14:1228969. doi: 10.3389/fphar.2023.1228969. eCollection 2023. Front Pharmacol. 2023. PMID: 37876728 Free PMC article. Review.
-
Prevalence and Significance of AGR2 Expression in Human Cancer.Cancer Med. 2024 Nov;13(21):e70407. doi: 10.1002/cam4.70407. Cancer Med. 2024. PMID: 39533806 Free PMC article.
-
The biochemical effects of carotenoids in orange carrots on the colonic proteome in a mouse model of diet-induced obesity.Front Nutr. 2024 Nov 11;11:1492380. doi: 10.3389/fnut.2024.1492380. eCollection 2024. Front Nutr. 2024. PMID: 39588046 Free PMC article.
-
The genetics of monogenic intestinal epithelial disorders.Hum Genet. 2023 May;142(5):613-654. doi: 10.1007/s00439-022-02501-5. Epub 2022 Nov 23. Hum Genet. 2023. PMID: 36422736 Free PMC article. Review.
References
-
- Crowley E., Warner N., Pan J., Khalouei S., Elkadri A., Fiedler K., Foong J., Turinsky A.L., Bronte-Tinkew D., Zhang S., Hu J., Tian D., Li D., Horowitz J., Siddiqui I., Upton J., Roifman C.M., Church P.C., Wall D.A., Ramani A.K., Kotlarz D., Klein C., Uhlig H., Snapper S.B., Gonzaga-Jauregui C., Paterson A.D., McGovern D.P.B., Brudno M., Walters T.D., Griffiths A.M., Muise A.M. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208–2220. - PMC - PubMed
-
- Hooper L.V., MacPherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

