In vitro selection of Giardia duodenalis for Albendazole resistance identifies a β-tubulin mutation at amino acid E198K
- PMID: 34237690
- PMCID: PMC8267433
- DOI: 10.1016/j.ijpddr.2021.05.003
In vitro selection of Giardia duodenalis for Albendazole resistance identifies a β-tubulin mutation at amino acid E198K
Abstract
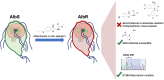
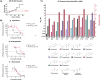
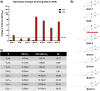
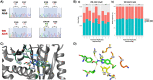
Benzimidazole-2-carbamate (BZ) compounds, including Albendazole (Alb), are one of just two drug classes approved to treat the gastrointestinal protist Giardia duodenalis. Benzimidazoles bind to the tubulin dimer interface overlapping the colchicine binding site (CBS) of β-tubulin, thereby inhibiting microtubule polymerisation and disrupting microtubule networks. These BZ compounds are widely used as anthelmintic, anti-fungal and anti-giardial drugs. However, in helminths and fungi, BZ-resistance is widespread and caused by specific point mutations primarily occurring at F167, E198 and F200 in β-tubulin isoform 1. BZ-resistance in Giardia is reported clinically and readily generated in vitro, with significant implications for Giardia control. In Giardia, BZ mode of action (MOA) and resistance mechanisms are presumed but not proven, and no mutations in β-tubulin have been reported in association with Alb resistance (AlbR). Herein, we undertook detailed in vitro drug-susceptibility screens of 13 BZ compounds and 7 Alb structural analogues in isogenic G. duodenalis isolates selected for AlbR and podophyllotoxin, another β-tubulin inhibitor, as well as explored cross-resistance to structurally unrelated, metronidazole (Mtz). AlbR lines exhibited co-resistance to many structural variants in the BZ-pharmacophore, and cross-resistance to podophyllotoxin. AlbR lines were not cross-resistant to Mtz, but MtzR lines had enhanced survival in Alb. Lastly, Alb analogues with longer thioether substituents had decreased potency against our AlbR lines. In silico modelling indicated the Alb-β-tubulin interaction in Giardia partially overlaps the CBS and corresponds to residues associated with BZ-resistance in helminths and fungi (F167, E198, F200). Sequencing of Giardia β-tubulin identified a single nucleotide polymorphism resulting in a mutation from glutamic acid to lysine at amino acid 198 (E198K). To our knowledge, this is the first β-tubulin mutation reported for protistan BZ-resistance. This study provides insight into BZ mode of action and resistance in Giardia, and presents a potential avenue for a genetic test for clinically resistance isolates.
Keywords: Albendazole; Benzimidazoles; Drug-resistance; Giardia duodenalis; Tubulin.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors listed have contributed to the research, have no conflict of interest or competing interests to declare, and have approved the manuscript for submission to IJPDDR. The funders of this study have had no role in its design, data collection and interpretation, and are listed in the manuscript’s acknowledgements.
Figures


Similar articles
-
Transcriptomic analysis of albendazole resistance in human diarrheal parasite Giardia duodenalis.Int J Parasitol Drugs Drug Resist. 2023 Aug;22:9-19. doi: 10.1016/j.ijpddr.2023.03.004. Epub 2023 Mar 24. Int J Parasitol Drugs Drug Resist. 2023. PMID: 37004489 Free PMC article.
-
Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in beta-tubulin.Microb Drug Resist. 1996 Fall;2(3):303-8. doi: 10.1089/mdr.1996.2.303. Microb Drug Resist. 1996. PMID: 9158790
-
In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression.Infect Genet Evol. 2009 Dec;9(6):1057-64. doi: 10.1016/j.meegid.2009.05.015. Epub 2009 May 27. Infect Genet Evol. 2009. PMID: 19481175
-
Biochemistry of benzimidazole resistance.Acta Trop. 1994 Mar;56(2-3):245-62. doi: 10.1016/0001-706x(94)90066-3. Acta Trop. 1994. PMID: 8203306 Review.
-
The binding and distribution of albendazole and its principal metabolites in Giardia duodenalis.J Vet Pharmacol Ther. 2000 Jun;23(3):113-20. doi: 10.1046/j.1365-2885.2000.00254.x. J Vet Pharmacol Ther. 2000. PMID: 11110097 Review.
Cited by
-
Antiparasitic activity of Colombian Amazon palm extracts against Giardia lamblia trophozoites: insights into cellular death mechanisms.Front Microbiol. 2025 Mar 19;16:1523880. doi: 10.3389/fmicb.2025.1523880. eCollection 2025. Front Microbiol. 2025. PMID: 40177476 Free PMC article.
-
Analysis of the role of acetylation in Giardia lamblia and the giardicidal potential of garcinol.Front Microbiol. 2025 Jan 3;15:1513053. doi: 10.3389/fmicb.2024.1513053. eCollection 2024. Front Microbiol. 2025. PMID: 39831116 Free PMC article.
-
Highly sensitive wastewater surveillance of SARS-CoV-2 variants by targeted next-generation amplicon sequencing provides early warning of incursion in Victoria, Australia.Appl Environ Microbiol. 2024 Aug 21;90(8):e0149723. doi: 10.1128/aem.01497-23. Epub 2024 Jul 16. Appl Environ Microbiol. 2024. PMID: 39012098 Free PMC article.
-
gdSir2.1 and gdSir2.3 are involved in albendazole resistance in Giardia duodenalis via regulation of the oxidative stress response.Int J Parasitol Drugs Drug Resist. 2025 Aug;28:100596. doi: 10.1016/j.ijpddr.2025.100596. Epub 2025 May 5. Int J Parasitol Drugs Drug Resist. 2025. PMID: 40373730 Free PMC article.
-
Enhancing Giardicidal Activity and Aqueous Solubility through the Development of "RetroABZ", a Regioisomer of Albendazole: In Vitro, In Vivo, and In Silico Studies.Int J Mol Sci. 2023 Oct 6;24(19):14949. doi: 10.3390/ijms241914949. Int J Mol Sci. 2023. PMID: 37834396 Free PMC article.
References
-
- Agarwal S.K., Sharma S., Bhaduri A.P., Katiyar J.C., Chatterjee R.K. Segregation of activity profile in benzimidazoles: effect of spacers at 5(6)-position of methyl benzimidazole-2-carbamates [1] Z. Naturforsch. C Biosci. 1993;48:829.
-
- Aguayo-Ortiz R., Mendez-Lucio O., Medina-Franco J.L., Castillo R., Yepez-Mulia L., Hernandez-Luis F., Hernandez-Campos A. Towards the identification of the binding site of benzimidazoles to beta-tubulin of Trichinella spiralis: insights from computational and experimental data. J. Mol. Graph. Model. 2013;41:12–19. - PubMed
-
- Aguayo-Ortiz R., Méndez-Lucio O., Romo-Mancillas A., Castillo R., Yépez-Mulia L., Medina-Franco J.L., Hernández-Campos A. Molecular basis for benzimidazole resistance from a novel β-tubulin binding site model. J. Mol. Graph. Model. 2013;45:26–37. - PubMed
-
- Ansell B.R., McConville M.J., Ma'ayeh S.Y., Dagley M.J., Gasser R.B., Svard S.G., Jex A.R. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015;33:888–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

