Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study
- PMID: 34238227
- PMCID: PMC8268596
- DOI: 10.1186/s12876-021-01856-9
Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study
Abstract
Background: Fecal microbiota transplantation (FMT) is a promising new strategy in the treatment of Inflammatory Bowel Disease, but long-term delivery systems are lacking. This randomized study was designed as a safety and feasibility study of long-term FMT in subjects with mild to moderate UC using frozen, encapsulated oral FMT (cFMT).
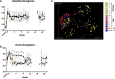
Methods: Subjects were randomized 1:1 to receive FMT induction by colonoscopy, followed by 12 weeks of daily oral administration of frozen encapsulated cFMT or sham therpay. Subjects were followed for 36 weeks and longitudenal clinical assessments included multiple subjective and objective markers of disease severity. Ribosomal 16S bacterial sequencing was used to assess donor-induced changes in the gut microbiota. Changes in T regulatory (Treg) and mucosal associated invariant T (MAIT) cell populations were evaluated by flow cytometry as an exploratory endpoint.
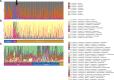
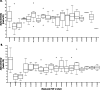
Results: Twelve subjects with active UC were randomized: 6 subjects completed the full 12-week course of FMT plus cFMT, and 6 subjects received sham treatment by colonic installation and longitudinal oral placebo capules. Chronic administration of cFMT was found to be safe and well-tolerated but home storage concerns exist. Protocol adherence was high, and none of the study subjects experienced FMT-associated treatment emergent adverse events. Two subjects that received cFMT achieved clinical remission versus none in the placebo group (95% CI = 0.38-infinity, p = 0.45). cFMT was associated with sustained donor-induced shifts in fecal microbial composition. Changes in MAIT cell cytokine production were observed in cFMT recipients and correlated with treatment response.
Conclusion: These pilot data suggest that daily encapsulated cFMT may extend the durability of index FMT-induced changes in gut bacterial community structure and that an association between MAIT cell cytokine production and clinical response to FMT may exist in UC populations. Oral frozen encapsulated cFMT is a promising FMT delivery system and may be preferred for longterm treatment strategies in UC and other chronic diseases but further evaluations will have to address home storage concerns. Larger trials should be done to explore the benefits of cFMT and to determine its long-term impacts on the colonic microbiome.
Trial registration: ClinicalTrials.gov (NCT02390726). Registered 17 March 2015, https://clinicaltrials.gov/ct2/show/NCT02390726?term=NCT02390726&draw=2&rank=1 .
Keywords: FMT; Fecal microbiota transplantation; IBD; Inflammatory bowel disease; MAIT cells; Microbiome; Microbiota; UC; Ulcerative colitis.
Conflict of interest statement
EJA, ZK and MS are cofounders of OpenBiome and Finch Therapeutics. ZK, MS, RJE, EV are employees of Finch Therapeutics. JWC and PLM consult for Finch Therapeutics. WFW, RJE, EV are employees of OpenBiome. GMM has research funding from Takeda Pharmaceuticals and is on the Scientific Advisory Board of Dignify Therapeutics.
Figures



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

