Real-world effectiveness of post-trastuzumab emtansine treatment in patients with HER2-positive, unresectable and/or metastatic breast cancer: a retrospective observational study (KBCSG-TR 1917)
- PMID: 34238257
- PMCID: PMC8268506
- DOI: 10.1186/s12885-021-08504-1
Real-world effectiveness of post-trastuzumab emtansine treatment in patients with HER2-positive, unresectable and/or metastatic breast cancer: a retrospective observational study (KBCSG-TR 1917)
Abstract
Background: Trastuzumab emtansine (T-DM1) is a second-line standard therapy for patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. Evidence regarding post-T-DM1 treatments is currently lacking. We evaluated the effectiveness of post-T-DM1 drug therapy in patients with HER2-positive, unresectable and/or metastatic breast cancer.
Methods: In this multicenter, retrospective, observational study, real-world clinical data of female patients with HER2-positive breast cancer who had a history of T-DM1 treatment were consecutively collected from five sites in Japan. We investigated the effectiveness of post-T-DM1 therapy by evaluating the real-world progression-free survival (rwPFS), time to treatment failure (TTF), overall survival (OS), objective response rate (ORR), and clinical benefit rate (CBR). Tumor response was assessed by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) guidelines. Subgroup and exploratory analyses according to background factors were also undertaken.
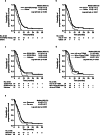
Results: Of the 205 patients who received T-DM1 treatment between 1 January 2014 and 31 December 2018, 128 were included in this study. Among the 128 patients analyzed, 105 (82%) patients received anti-HER2 therapy and 23 (18%) patients received regimens without anti-HER2 therapy. Median (95% confidence interval [CI]) rwPFS, TTF, and OS were 5.7 (4.8-6.9) months, 5.6 (4.6-6.4) months, and 22.8 (18.2-32.4) months, respectively. CBR and ORR (95% CI) were 48% (38.8-56.7) and 23% (15.1-31.4), respectively. Cox-regression analysis showed that an ECOG PS score of 0, a HER2 immunohistochemistry score of 3+, recurrent type, ≥12 month duration of T-DM1 therapy, and anti-HER2 therapy were independent variables for rwPFS. An exploratory subgroup analysis of regimens after T-DM1 showed that those with anti-HER2 therapy had a median rwPFS of 6.3 and those without anti-HER2 therapy had a median rwPFS of 4.8 months.
Conclusions: In the real-world setting in Japan, several post-T-DM1 regimens for patients with unresectable and/or metastatic HER2-positive breast cancer, including continuation of anti-HER2 therapy, showed some effectiveness; however, this effectiveness was insufficient. Novel therapeutic options are still needed for further improvement of PFS and OS in later treatment settings.
Trial registration: UMIN000038296 ; registered on 15 October 2019.
Keywords: HER2-positive; KBCSG-TR 1917; Retrospective observational study; T-DM1/trastuzumab emtansine; Unresectable and/or metastatic breast cancer.
Conflict of interest statement
TN has received speakers’ bureau fees from Chugai Pharma, AstraZeneca, Eli Lilly, Novartis, Takeda, Taiho Pharmaceutical, and Daiichi Sankyo. TYo has received consulting or advisory fees from Daiichi Sankyo and speakers’ bureau fees from Chugai Pharma and Novartis. NM has received honoraria from Chugai Pharma, AstraZeneca, Pfizer, Eisai, Eli Lilly, and Takeda; research funding from Chugai Pharma, AstraZeneca, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Eli Lilly, Eisai, and Daiichi Sankyo; and has a leadership role at the Japan Breast Cancer Research Group. SO has received honoraria from Chugai Pharma, Pfizer, and Eisai. SJK has received honoraria and research funding from Daiichi Sankyo. MT has received honoraria from AstraZeneca, Eisai, Pfizer, Eli Lilly, Chugai Pharma, and Nippon Kayaku; and research funding from Taiho Pharmaceutical, Kyowa Hakko Kirin, Eisai, and Nippon Kayaku. TT is an employee of Daiichi Sankyo. NY has an immediate family member who is an employee of and has owned stock of held ownership interest in Bayer Yakuhin. NK, HK, HY, and TYa have no conflicting interests to declare.
Figures


References
-
- Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–6. 10.1200/JCO.2009.23.2025. - PMC - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. 10.1126/science.2470152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

