Acute pre-operative ibuprofen improves cognition in a rat model for postoperative cognitive dysfunction
- PMID: 34238316
- PMCID: PMC8265047
- DOI: 10.1186/s12974-021-02206-y
Acute pre-operative ibuprofen improves cognition in a rat model for postoperative cognitive dysfunction
Abstract
Background: Inflammation is considered a key factor in the development of postoperative cognitive dysfunction (POCD). Therefore, we hypothesized that pre-operative anti-inflammatory treatment with ibuprofen would inhibit POCD in our rat-model.
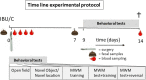
Methods: Male Wistar rats of 3 or 23 months old received a single injection of ibuprofen (15 mg/kg i.p.) or were control handled before abdominal surgery. Timed blood and fecal samples were collected for analyses of inflammation markers and gut microbiome changes. Behavioral testing was performed from 9 to 14 days after surgery, in the open field, novel object- and novel location-recognition tests and Morris water maze. Neuroinflammation and neurogenesis were assessed by immune histochemistry after sacrifice on postoperative day 14.
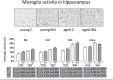
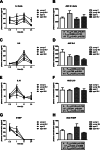
Results: Ibuprofen improved short-term spatial memory in the novel location recognition test, and increased hippocampal neurogenesis. However, these effects were associated with increased hippocampal microglia activity. Whereas plasma cytokine levels (IL1-β, IL6, IL10, and TNFα) were not significantly affected, VEGF levels increased and IFABP levels decreased after ibuprofen. Long-term memory in the Morris water maze was not significantly improved by ibuprofen. The gut microbiome was neither significantly affected by surgery nor by ibuprofen treatment. In general, effects in aged rats appeared similar to those in young rats, though less pronounced.
Conclusion: A single injection of ibuprofen before surgery improved hippocampus-associated short-term memory after surgery and increased neurogenesis. However, this favorable outcome seemed not attributable to inhibition of (neuro)inflammation. Potential contributions of intestinal and blood-brain barrier integrity need further investigation. Although less pronounced compared to young rats, effects in aged rats indicate that even elderly individuals could benefit from ibuprofen treatment.
Keywords: Cognition; Ibuprofen; Inflammation; Neuroinflammation; Postoperative cognitive dysfunction.
Conflict of interest statement
The authors have no competing interests to declare.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

