Easy and quick (EQ) sperm freezing method for urgent preservation of mouse strains
- PMID: 34239008
- PMCID: PMC8266870
- DOI: 10.1038/s41598-021-93604-y
Easy and quick (EQ) sperm freezing method for urgent preservation of mouse strains
Abstract
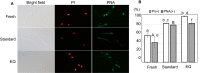
Cryopreservation of mouse spermatozoa is widely used for the efficient preservation and safe transport of valuable mouse strains. However, the current cryopreservation method requires special containers (plastic straws), undefined chemicals (e.g., skim milk), liquid nitrogen, and expertise when handling sperm suspensions. Here, we report an easy and quick (EQ) sperm freezing method. The main procedure consists of only one step: dissecting a single cauda epididymis in a microtube containing 20% raffinose solution, which is then stored in a -80 °C freezer. The frozen-thawed spermatozoa retain practical fertilization rates after 1 (51%) or even 3 months (25%) with the C57BL/6 J strain, the most sensitive strain for sperm freezing. More than half of the embryos thus obtained developed into offspring after embryo transfer. Importantly, spermatozoa stored at -80 °C can be transferred into liquid nitrogen for indefinite storage. As far as we know, our EQ method is the easiest and quickest method for mouse sperm freezing and should be applicable in all laboratories without expertise in sperm cryopreservation. This technique can help avoid the loss of irreplaceable strains because of closure of animal rooms in emergency situations such as unexpected microbiological contamination or social emergencies such as the COVID-19 threat.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Thornton CE, Brown SDM, Glenister PH. Large numbers of mice established by in vitro fertilization with cryopreserved spermatozoa: implications and applications for genetic resource banks, mutagenesis screens, and mouse backcrosses. Mamm. Genome. 1999;10:987–992. doi: 10.1007/s003359901145. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

