Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling
- PMID: 34239070
- PMCID: PMC8486800
- DOI: 10.1038/s41422-021-00528-3
Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling
Abstract
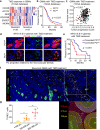
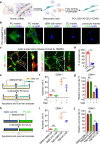
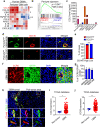
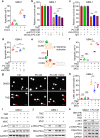
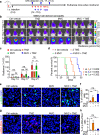
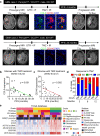
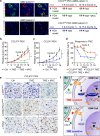
Glioblastoma (GBM) is a prevalent and highly lethal form of glioma, with rapid tumor progression and frequent recurrence. Excessive outgrowth of pericytes in GBM governs the ecology of the perivascular niche, but their function in mediating chemoresistance has not been fully explored. Herein, we uncovered that pericytes potentiate DNA damage repair (DDR) in GBM cells residing in the perivascular niche, which induces temozolomide (TMZ) chemoresistance. We found that increased pericyte proportion correlates with accelerated tumor recurrence and worse prognosis. Genetic depletion of pericytes in GBM xenografts enhances TMZ-induced cytotoxicity and prolongs survival of tumor-bearing mice. Mechanistically, C-C motif chemokine ligand 5 (CCL5) secreted by pericytes activates C-C motif chemokine receptor 5 (CCR5) on GBM cells to enable DNA-dependent protein kinase catalytic subunit (DNA-PKcs)-mediated DDR upon TMZ treatment. Disrupting CCL5-CCR5 paracrine signaling through the brain-penetrable CCR5 antagonist maraviroc (MVC) potently inhibits pericyte-promoted DDR and effectively improves the chemotherapeutic efficacy of TMZ. GBM patient-derived xenografts with high CCL5 expression benefit from combined treatment with TMZ and MVC. Our study reveals the role of pericytes as an extrinsic stimulator potentiating DDR signaling in GBM cells and suggests that targeting CCL5-CCR5 signaling could be an effective therapeutic strategy to improve chemotherapeutic efficacy against GBM.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

