Anti-CD22 CAR-T Cell Therapy as a Salvage Treatment in B Cell Malignancies Refractory or Relapsed After Anti-CD19 CAR-T therapy
- PMID: 34239307
- PMCID: PMC8259947
- DOI: 10.2147/OTT.S312904
Anti-CD22 CAR-T Cell Therapy as a Salvage Treatment in B Cell Malignancies Refractory or Relapsed After Anti-CD19 CAR-T therapy
Abstract
Background: To observe efficacy of the anti-CD22 chimeric antigen receptor modified (anti-CD22-CAR) T cell salvage therapy in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and B cell acute lymphoid leukemia (B-ALL) patients whose disease did not reach CR or progressed again after anti-CD19-CAR T cell therapy.
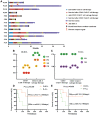
Methods: In our study, seven R/R DLBCL patients reached stable disease (SD) or progression of disease (PD) after their anti-CD19-CAR T cell therapy. Only three in all the six R/R B-ALL patients obtained complete response (CR)/CR with incomplete count recovery (Cri) in their anti-CD19-CAR T cell therapy, but they relapsed again in the following three, six and one months. Then, all these thirteen R/R DLBCL and B-ALL patients received anti-CD22 CAR-T cell salvage therapy because their disease did not reach CR or progressed again.
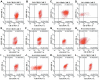
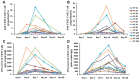
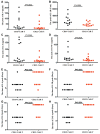
Results: Four R/R DLBCL patients obtained CR, while two R/R DLBCL patients achieved PR and one patient achieved SD. But only two R/R B-ALL patients obtained Cri in their anti-CD22 CAR-T cell salvage therapy. The overall survival (OS) of R/R DLBCL patients after the anti-CD22 CAR-T cell therapy was 6.142±3.395 months until August 31, 2020. There was no different of the median expansion peaks of the two kinds of CAR T cells (P=0.920). The time of anti-CD22-CAR T cell proportion peak days was later than that of the time of anti-CD19-CAR T cell peak days post infusion (P=0.022). Their cytokine release syndrome (CRS) was graded 2-4 in their anti-CD19-CAR T cell therapy, while the notable CRS was graded 1-2 in their anti-CD22-CAR T cell therapy. But there was no difference in the CRS and the immune effect or cell associated neurotoxic syndrome (ICANS) grades in the two kinds of therapies. And there was no difference in the hematological toxicity grades in the two kinds of therapies.
Conclusion: The anti-CD22-CAR T cell salvage therapy is highly effective in R/R DLBCL patients than in R/R B-ALL patients who failed in anti-CD19-CAR T cell therapy before. We need to expand the number of R/R DLBCL or B-ALL patients and continue to observe.
Trial registration: ChiCTR-ONN-16009862 and ChiCTR1800019298.
Keywords: CAR; acute lymphoblastic leukemia; anti-CD19; anti-CD22; chimeric antigen receptor; diffuse large B-cell lymphoma.
© 2021 Zhu et al.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

