BMP and Notch Signaling Pathways differentially regulate Cardiomyocyte Proliferation during Ventricle Regeneration
- PMID: 34239346
- PMCID: PMC8241734
- DOI: 10.7150/ijbs.59648
BMP and Notch Signaling Pathways differentially regulate Cardiomyocyte Proliferation during Ventricle Regeneration
Abstract
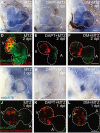
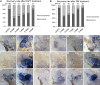
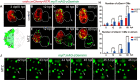
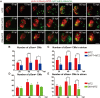
Adult mammalian hearts show limited capacity to proliferate after injury, while zebrafish are capable to completely regenerate injured hearts through the proliferation of spared cardiomyocytes. BMP and Notch signaling pathways have been implicated in cardiomyocyte proliferation during zebrafish heart regeneration. However, the molecular mechanism underneath this process as well as the interaction between these two pathways remains to be further explored. In this study we showed BMP signaling was activated after ventricle ablation and acted epistatic downstream of Notch signaling. Inhibition of both signaling pathways differentially influenced ventricle regeneration and cardiomyocyte proliferation, as revealed by time-lapse analysis using a cardiomyocyte-specific FUCCI (fluorescent ubiquitylation-based cell cycle indicator) system. Further experiments revealed that inhibition of BMP and Notch signaling led to cell-cycle arrest at different phases. Overall, our results shed light on the interaction between BMP and Notch signaling pathways and their functions in cardiomyocyte proliferation during cardiac regeneration.
Keywords: BMP signaling; Notch signaling; cardiomyocyte proliferation; cell-cycle arrest; heart regeneration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Journal of the American College of Cardiology. 2007;50:2173–95. - PubMed
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP. et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. - PubMed
-
- Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

