Spinal Cord Injury Causes Reduction of Galanin and Gastrin Releasing Peptid e mRNA Expression in the Spinal Ejaculation Generator of Male Rats
- PMID: 34239493
- PMCID: PMC8258150
- DOI: 10.3389/fneur.2021.670536
Spinal Cord Injury Causes Reduction of Galanin and Gastrin Releasing Peptid e mRNA Expression in the Spinal Ejaculation Generator of Male Rats
Abstract
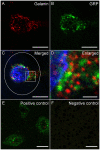
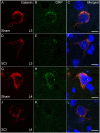
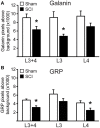
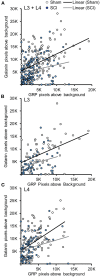
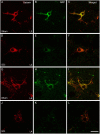
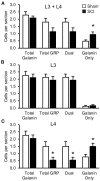
Spinal cord injury (SCI) in men is commonly associated with sexual dysfunction, including anejaculation, and chronic mid-thoracic contusion injury in male rats also impairs ejaculatory reflexes. Ejaculation is controlled by a spinal ejaculation generator consisting of a population of lumbar spinothalamic (LSt) neurons that control ejaculation through release of four neuropeptides including galanin and gastrin releasing peptide (GRP) onto lumbar and sacral autonomic and motor nuclei. It was recently demonstrated that spinal contusion injury in male rats caused reduction of GRP-immunoreactivity, but not galanin-immunoreactivity in LSt cells, indicative of reduced GRP peptide levels, but inconclusive results for galanin. The current study further tests the hypothesis that contusion injury causes a disruption of GRP and galanin mRNA in LSt cells. Male rats received mid-thoracic contusion injury and galanin and GRP mRNA were visualized 8 weeks later in the lumbar spinal cord using fluorescent in situ hybridization. Spinal cord injury significantly reduced GRP and galanin mRNA in LSt cells. Galanin expression was higher in LSt cells compared to GRP. However, expression of the two transcripts were positively correlated in LSt cells in both sham and SCI animals, suggesting that expression for the two neuropeptides may be co-regulated. Immunofluorescent visualization of galanin and GRP peptides demonstrated a significant reduction in GRP-immunoreactivity, but not galanin in LSt cells, confirming the previous observations. In conclusion, SCI reduced GRP and galanin expression in LSt cells with an apparent greater impact on GRP peptide levels. GRP and galanin are both essential for triggering ejaculation and thus such reduction may contribute to ejaculatory dysfunction following SCI in rats.
Keywords: anejaculation; contusion spinal injury; lumbar spinal cord; sexual dysfunction; urogenital.
Copyright © 2021 Wiggins, Sledd and Coolen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Neurons for Ejaculation and Factors Affecting Ejaculation.Biology (Basel). 2022 Apr 29;11(5):686. doi: 10.3390/biology11050686. Biology (Basel). 2022. PMID: 35625414 Free PMC article. Review.
-
Chronic Spinal Cord Injury Reduces Gastrin-Releasing Peptide in the Spinal Ejaculation Generator in Male Rats.J Neurotrauma. 2019 Dec 15;36(24):3378-3393. doi: 10.1089/neu.2019.6509. Epub 2019 Jul 10. J Neurotrauma. 2019. PMID: 31111794 Free PMC article.
-
Activation of gastrin-releasing peptide receptors in the lumbosacral spinal cord is required for ejaculation in male rats.J Sex Med. 2012 May;9(5):1303-18. doi: 10.1111/j.1743-6109.2012.02688.x. Epub 2012 Mar 16. J Sex Med. 2012. PMID: 22429708
-
Activation of galanin and cholecystokinin receptors in the lumbosacral spinal cord is required for ejaculation in male rats.Eur J Neurosci. 2017 Mar;45(6):846-858. doi: 10.1111/ejn.13515. Epub 2017 Jan 24. Eur J Neurosci. 2017. PMID: 28002640
-
[Spinal gastrin-releasing peptide system mediates sexual function of males: advances in studies].Zhonghua Nan Ke Xue. 2014 Jun;20(6):554-7. Zhonghua Nan Ke Xue. 2014. PMID: 25029865 Review. Chinese.
Cited by
-
Sexual Experience Induces the Expression of Gastrin-Releasing Peptide and Oxytocin Receptors in the Spinal Ejaculation Generator in Rats.Int J Mol Sci. 2021 Sep 26;22(19):10362. doi: 10.3390/ijms221910362. Int J Mol Sci. 2021. PMID: 34638701 Free PMC article.
-
Neurons for Ejaculation and Factors Affecting Ejaculation.Biology (Basel). 2022 Apr 29;11(5):686. doi: 10.3390/biology11050686. Biology (Basel). 2022. PMID: 35625414 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

