FMRFamide-Like Peptide 22 Influences the Head Movement, Host Finding, and Infection of Heterodera glycines
- PMID: 34239524
- PMCID: PMC8258376
- DOI: 10.3389/fpls.2021.673354
FMRFamide-Like Peptide 22 Influences the Head Movement, Host Finding, and Infection of Heterodera glycines
Abstract
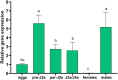
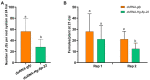
The FMRFamide-like peptides (FLPs) represent the largest family of nematode neuropeptides and are involved in multiple parasitic activities. The immunoreactivity to FMRFamide within the nervous system of Heterodera glycines, the most economically damaging parasite of soybean [Glycine max L. (Merr)], has been reported in previous research. However, the family of genes encoding FLPs of H. glycines were not identified and functionally characterized. In this study, an FLP encoding gene Hg-flp-22 was cloned from H. glycines, and its functional characterization was uncovered by using in vitro RNA interference and application of synthetic peptides. Bioinformatics analysis showed that flp-22 is widely expressed in multiple nematode species, where they encode the highly conserved KWMRFamide motifs. Quantitative real-time (qRT)-PCR results revealed that Hg-flp-22 was highly expressed in the infective second-stage juveniles (J2s) and adult males. Silencing of Hg-flp-22 resulted in the reduced movement of J2s to the host root and reduced penetration ability, as well as a reduction in their subsequent number of females. Behavior and infection assays demonstrated that application of synthetic peptides Hg-FLP-22b (TPQGKWMRFa) and Hg-FLP-22c (KMAIEGGKWVRFa) significantly increased the head movement frequency and host invasion abilities in H. glycines but not in Meloidogyne incognita. In addition, the number of H. glycines females on the host roots was found to be significantly higher in Hg-FLP-22b treated nematodes than the ddH2O-treated control J2s. These results presented in this study elucidated that Hg-flp-22 plays a role in regulating locomotion and infection of H. glycines. This suggests the potential of FLP signaling as putative control targets for H. glycines in soybean production.
Keywords: FMRFamide-like peptides; Heterodera glycines; neuropeptides; soybean; soybean cyst nematode.
Copyright © 2021 You, Pan, Wang, Wang and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Atkinson H. J., Isaac R. E., Harris P. D., Sharpe C. M. (1988). FMRFamide-like immunoreactivity within the nervous-system of the nematodes panagrellus-redivius, Caenorhabditis elegans and Heterodera glycines. J. Zool. 216, 663–671. 10.1111/j.1469-7998.1988.tb02464.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

