Autophagic Degradation of Gasdermin D Protects against Nucleus Pulposus Cell Pyroptosis and Retards Intervertebral Disc Degeneration In Vivo
- PMID: 34239691
- PMCID: PMC8238599
- DOI: 10.1155/2021/5584447
Autophagic Degradation of Gasdermin D Protects against Nucleus Pulposus Cell Pyroptosis and Retards Intervertebral Disc Degeneration In Vivo
Abstract
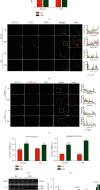
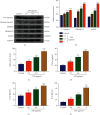
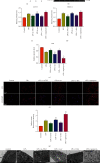
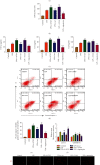
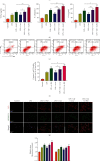
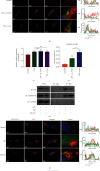
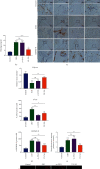
Intervertebral disc degeneration (IDD) is the primary culprit of low back pain and renders heavy social burden worldwide. Pyroptosis is a newly discovered form of programmed cell death, which is also involved in nucleus pulposus (NP) cells during IDD progression. Moderate autophagy activity is critical for NP cell survival, but its relationship with pyroptosis remains unknown. This study is aimed at investigating the relationship between autophagy and pyroptotic cell death. The pyroptosis executor N-terminal domain of gasdermin D (GSDMD-N) and inflammation-related proteins were measured in lipopolysaccharide- (LPS-) treated human NP cells. Inhibition of autophagy by siRNA transfection and chemical drugs aggravated human NP cell pyroptosis. Importantly, we found that the autophagy-lysosome pathway and not the proteasome pathway mediated the degradation of GSDMD-N as lysosome dysfunction promoted the accumulation of cytoplasmic GSDMD-N. Besides, P62/SQSTM1 colocalized with GSDMD-N and mediated its degradation. The administration of the caspase-1 inhibitor VX-765 could reduce cell pyroptosis as confirmed in a rat disc IDD model in vivo, whereas ATG5 knockdown significantly accelerated the progression of IDD. In conclusion, our study indicated that autophagy protects against LPS-induced human NP cell pyroptosis via a P62/SQSTM1-mediated degradation mechanism and the inhibition of pyroptosis retards IDD progression in vivo. These findings deepen the understanding of IDD pathogenesis and hold implications in unraveling therapeutic targets for IDD treatment.
Copyright © 2021 Zhiwei Liao et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ocifisertib alleviates the gasdermin D-independent pyroptosis of nucleus pulposus cells by targeting GSDME.Sci Rep. 2025 Apr 17;15(1):13280. doi: 10.1038/s41598-025-98283-7. Sci Rep. 2025. PMID: 40247083 Free PMC article.
-
SOX4 accelerates intervertebral disc degeneration via EZH2/NRF2 pathway in response to mitochondrial ROS-dependent NLRP3 inflammasome activation in nucleus pulposus cells.J Transl Med. 2025 Apr 3;23(1):395. doi: 10.1186/s12967-024-05913-1. J Transl Med. 2025. PMID: 40181390 Free PMC article.
-
TIGAR attenuates intervertebral disc degeneration via autophagy-mediated Keap1 degradation and Nrf2 nuclear translocation to suppress nucleus pulposus pyroptosis.Cell Signal. 2025 Aug;132:111856. doi: 10.1016/j.cellsig.2025.111856. Epub 2025 May 7. Cell Signal. 2025. PMID: 40345508
-
Role of Pyroptosis in Intervertebral Disc Degeneration and Its Therapeutic Implications.Biomolecules. 2022 Dec 2;12(12):1804. doi: 10.3390/biom12121804. Biomolecules. 2022. PMID: 36551232 Free PMC article. Review.
-
Roles of pyroptosis in intervertebral disc degeneration.Pathol Res Pract. 2023 Aug;248:154685. doi: 10.1016/j.prp.2023.154685. Epub 2023 Jul 16. Pathol Res Pract. 2023. PMID: 37494803 Review.
Cited by
-
Role of Caspase Family in Intervertebral Disc Degeneration and Its Therapeutic Prospects.Biomolecules. 2022 Aug 4;12(8):1074. doi: 10.3390/biom12081074. Biomolecules. 2022. PMID: 36008968 Free PMC article. Review.
-
Ferroptosis and pyroptosis are connected through autophagy: a new perspective of overcoming drug resistance.Mol Cancer. 2025 Jan 17;24(1):23. doi: 10.1186/s12943-024-02217-2. Mol Cancer. 2025. PMID: 39825385 Free PMC article. Review.
-
Syntaxin17 Restores Lysosomal Function and Inhibits Pyroptosis Caused by Acinetobacter baumannii.J Microbiol. 2024 Apr;62(4):315-325. doi: 10.1007/s12275-024-00109-0. Epub 2024 Mar 7. J Microbiol. 2024. PMID: 38451450
-
Regulated cell death: Implications for intervertebral disc degeneration and therapy.J Orthop Translat. 2022 Nov 5;37:163-172. doi: 10.1016/j.jot.2022.10.009. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36380883 Free PMC article. Review.
-
Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis.Cell Mol Immunol. 2023 Jan;20(1):51-64. doi: 10.1038/s41423-022-00956-0. Epub 2022 Nov 30. Cell Mol Immunol. 2023. PMID: 36447031 Free PMC article.
References
-
- Kerr G. J., Veras M. A., Kim M. K., Séguin C. A. Decoding the intervertebral disc: unravelling the complexities of cell phenotypes and pathways associated with degeneration and mechanotransduction. Seminars in Cell & Developmental Biology. 2017;62:94–103. doi: 10.1016/j.semcdb.2016.05.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

