Real-World Data on Clinical Outcomes of Patients with Liver Cancer: A Prospective Validation of the National Cancer Centre Singapore Consensus Guidelines for the Management of Hepatocellular Carcinoma
- PMID: 34239809
- PMCID: PMC8237792
- DOI: 10.1159/000514400
Real-World Data on Clinical Outcomes of Patients with Liver Cancer: A Prospective Validation of the National Cancer Centre Singapore Consensus Guidelines for the Management of Hepatocellular Carcinoma
Abstract
Introduction: Real-world management of patients with hepatocellular carcinoma (HCC) is crucially challenging in the current rapidly evolving clinical environment which includes the need for respecting patient preferences and autonomy. In this context, regional/national treatment guidelines nuanced to local demographics have increasing importance in guiding disease management. We report here real-world data on clinical outcomes in HCC from a validation of the Consensus Guidelines for HCC at the National Cancer Centre Singapore (NCCS).
Method: We evaluated the NCCS guidelines using prospectively collected real-world data, comparing the efficacy of treatment received using overall survival (OS) and progression-free survival (PFS). Treatment outcomes were also independently evaluated against 2 external sets of guidelines, the Barcelona Clinic Liver Cancer (BCLC) and Hong Kong Liver Cancer (HKLC).
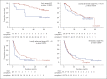
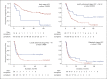
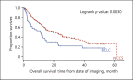
Results: Overall treatment compliance to the NCCS guidelines was 79.2%. Superior median OS was observed in patients receiving treatment compliant with NCCS guidelines for early (nonestimable vs. 23.5 months p < 0.0001), locally advanced (28.1 vs. 22.2 months p = 0.0216) and locally advanced with macrovascular invasion (10.3 vs. 3.3 months p = 0.0013) but not for metastatic HCC (8.1 vs. 6.8 months p = 0.6300), but PFS was similar. Better clinical outcomes were seen in BCLC C patients who received treatment compliant with NCCS guidelines than in patients with treatment only allowed by BCLC guidelines (median OS 14.2 vs. 7.4 months p = 0.0002; median PFS 6.1 vs. 4.0 months p = 0.0286). Clinical outcomes were, however, similar for patients across all HKLC stages receiving NCCS-recommended treatment regardless of whether their treatment was allowed by HKLC.
Conclusion: The high overall compliance rate and satisfactory clinical outcomes of patients managed according to the NCCS guidelines confirm its validity. This validation using real-world data considers patient and treating clinician preferences, thus providing a realistic analysis of the usefulness of the NCCS guidelines when applied in the clinics.
Keywords: Hepatocellular carcinoma; Liver cancer; Practice guidelines; Real-world data.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
Pierce K.H. Chow declares the following conflicts of interest: Editorial Board Member of Liver Cancer, Consulting or Advisory Role: Sirtex Medical, Bayer, Roche, New B Innovation, MSD, BTG PLC, Eisai, Abbott, IQVIA, Genentech, L.E.K Consulting, Astra Zeneca, Guerbet, Ipsen, OncoSil Medical, and Bristol-Myers Squibb; Speakers' Bureau: Sirtex Medical; and Research Funding: Sirtex Medical (Inst), New B Innovation (Inst), and IQVIA (Inst).
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. - PubMed
-
- Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015 Jan;61((1)):184–90. - PubMed
-
- Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 2018 May 23;38:262–79. - PubMed
-
- Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29((3)):339–64. - PubMed
LinkOut - more resources
Full Text Sources

