Iron Chelator Transmetalative Approach to Inhibit Human Ribonucleotide Reductase
- PMID: 34240081
- PMCID: PMC8243325
- DOI: 10.1021/jacsau.1c00078
Iron Chelator Transmetalative Approach to Inhibit Human Ribonucleotide Reductase
Abstract
Efforts directed at curtailing the bioavailability of intracellular iron could lead to the development of broad-spectrum anticancer drugs given the metal's role in cancer proliferation and metastasis. Human ribonucleotide reductase (RNR), the key enzyme responsible for synthesizing the building blocks of DNA replication and repair, depends on Fe binding at its R2 subunit to activate the catalytic R1 subunit. This work explores an intracellular iron chelator transmetalative approach to inhibit RNR using the titanium(IV) chemical transferrin mimetic (cTfm) compounds Ti(HBED) and Ti(Deferasirox)2. Whole-cell EPR studies reveal that the compounds can effectively attenuate RNR activity though seemingly causing different changes to the labile iron pool that may account for differences in their potency against cells. Studies of Ti(IV) interactions with the adenosine nucleotide family at pH 7.4 reveal strong metal binding and extensive phosphate hydrolysis, which suggest the capacity of the metal to disturb the nucleotide substrate pool of the RNR enzyme. By decreasing intracellular Fe bioavailability and altering the nucleotide substrate pool, the Ti cTfm compounds could inhibit the activity of the R1 and R2 subunits of RNR. The compounds arrest the cell cycle in the S phase, indicating suppressed DNA replication, and induce apoptotic cell death. Cotreatment cell viability studies with cisplatin and Ti(Deferasirox)2 reveal a promising synergism between the compounds that is likely owed to their distinct but complementary effect on DNA replication.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
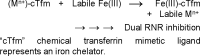






References
-
- Weinberg R.The biology of cancer, 2nd ed.; Garland Science: New York, 2013; p 960.
-
- Lane D. J. R.; Merlot A. M.; Huang M. L. H.; Bae D. H.; Jansson P. J.; Sahni S.; Kalinowski D. S.; Richardson D. R. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta, Mol. Cell Res. 2015, 1853 (5), 1130–1144. 10.1016/j.bbamcr.2015.01.021. - DOI - PubMed
