Metabolic derangements are associated with impaired glucose delivery following traumatic brain injury
- PMID: 34240124
- PMCID: PMC8677561
- DOI: 10.1093/brain/awab255
Metabolic derangements are associated with impaired glucose delivery following traumatic brain injury
Abstract
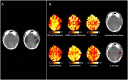
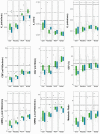
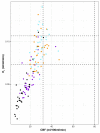
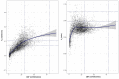
Metabolic derangements following traumatic brain injury are poorly characterized. In this single-centre observational cohort study we combined 18F-FDG and multi-tracer oxygen-15 PET to comprehensively characterize the extent and spatial pattern of metabolic derangements. Twenty-six patients requiring sedation and ventilation with intracranial pressure monitoring following head injury within a Neurosciences Critical Care Unit, and 47 healthy volunteers were recruited. Eighteen volunteers were excluded for age over 60 years (n = 11), movement-related artefact (n = 3) or physiological instability during imaging (n = 4). We measured cerebral blood flow, blood volume, oxygen extraction fraction, and 18F-FDG transport into the brain (K1) and its phosphorylation (k3). We calculated oxygen metabolism, 18F-FDG influx rate constant (Ki), glucose metabolism and the oxygen/glucose metabolic ratio. Lesion core, penumbra and peri-penumbra, and normal-appearing brain, ischaemic brain volume and k3 hotspot regions were compared with plasma and microdialysis glucose in patients. Twenty-six head injury patients, median age 40 years (22 male, four female) underwent 34 combined 18F-FDG and oxygen-15 PET at early, intermediate, and late time points (within 24 h, Days 2-5, and Days 6-12 post-injury; n = 12, 8, and 14, respectively), and were compared with 20 volunteers, median age 43 years (15 male, five female) who underwent oxygen-15, and nine volunteers, median age 56 years (three male, six female) who underwent 18F-FDG PET. Higher plasma glucose was associated with higher microdialysate glucose. Blood flow and K1 were decreased in the vicinity of lesions, and closely related when blood flow was <25 ml/100 ml/min. Within normal-appearing brain, K1 was maintained despite lower blood flow than volunteers. Glucose utilization was globally reduced in comparison with volunteers (P < 0.001). k3 was variable; highest within lesions with some patients showing increases with blood flow <25 ml/100 ml/min, but falling steeply with blood flow lower than 12 ml/100 ml/min. k3 hotspots were found distant from lesions, with k3 increases associated with lower plasma glucose (Rho -0.33, P < 0.001) and microdialysis glucose (Rho -0.73, P = 0.02). k3 hotspots showed similar K1 and glucose metabolism to volunteers despite lower blood flow and oxygen metabolism (P < 0.001, both comparisons); oxygen extraction fraction increases consistent with ischaemia were uncommon. We show that glucose delivery was dependent on plasma glucose and cerebral blood flow. Overall glucose utilization was low, but regional increases were associated with reductions in glucose availability, blood flow and oxygen metabolism in the absence of ischaemia. Clinical management should optimize blood flow and glucose delivery and could explore the use of alternative energy substrates.
Keywords: PET; cerebral blood flow; glucose metabolism; microdialysis; traumatic brain injury.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Coles JP, Cunningham AS, Salvador R, et al.Early metabolic characteristics of lesion and nonlesion tissue after head injury. J Cereb Blood Flow Metab. 2009;29(5):965–975. - PubMed
-
- Hattori N, Huang SC, Wu HM, et al.Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J Nucl Med. 2004;45(5):775–783. - PubMed
-
- Wu HM, Huang SC, Hattori N, et al.Subcortical white matter metabolic changes remote from focal hemorrhagic lesions suggest diffuse injury after human traumatic brain injury. Neurosurgery. 2004;55(6):1306–1315; discussion 1316–1317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

