Multiparametric magnetic resonance imaging to characterize cabotegravir long-acting formulation depot kinetics in healthy adult volunteers
- PMID: 34240449
- PMCID: PMC9290983
- DOI: 10.1111/bcp.14977
Multiparametric magnetic resonance imaging to characterize cabotegravir long-acting formulation depot kinetics in healthy adult volunteers
Abstract
Aim: Cabotegravir long-acting (LA) intramuscular (IM) injection is being investigated for HIV preexposure prophylaxis due to its potent antiretroviral activity and infrequent dosing requirement. A subset of healthy adult volunteers participating in a Phase I study assessing cabotegravir tissue pharmacokinetics underwent serial magnetic resonance imaging (MRI) to assess drug depot localization and kinetics following a single cabotegravir LA IM targeted injection.
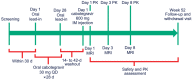
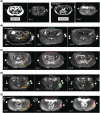
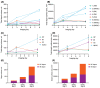
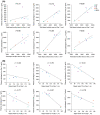
Methods: Eight participants (four men, four women) were administered cabotegravir LA 600 mg under ultrasonographic-guided injection targeting the gluteal muscles. MRI was performed to determine injection-site location in gluteal muscle (IM), subcutaneous (SC) adipose tissue and combined IM/SC compartments, and to quantify drug depot characteristics, including volume and surface area, on Days 1 (≤2 hours postinjection), 3 and 8. Linear regression analysis examined correlations between MRI-derived parameters and plasma cabotegravir exposure metrics, including maximum observed concentration (Cmax ) and partial area under the concentration-time curve (AUC) through Weeks 4 and 8.
Results: Cabotegravir LA depot locations varied by participant and were identified in the IM compartment (n = 2), combined IM/SC compartments (n = 4), SC compartment (n = 1) and retroperitoneal cavity (n = 1). Although several MRI parameter and exposure metric correlations were determined, total depot surface area on Day 1 strongly correlated with plasma cabotegravir concentration at Days 3 and 8, Cmax and partial AUC through Weeks 4 and 8.
Conclusion: MRI clearly delineated cabotegravir LA injection-site location and depot kinetics in healthy adults. Although injection-site variability was observed, drug depot surface area correlated with both plasma Cmax and partial AUC independently of anatomical distribution.
Keywords: HIV/AIDS; MRI; antiretrovirals; cabotegravir; pharmacokinetics-pharmacodynamic.
© 2021 ViiV Healthcare. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
B.M.J., V.D., P.G., R.J., J.S.S., K.B., K.H., S.F. and M.K.G. are employees of and own stock in GlaxoSmithKline. E.J.F. and E.D.W. received grant funding to their institution from GlaxoSmithKline. S.L. received personal fees from GlaxoSmithKline during the conduct of the study and from GE Healthcare outside of the submitted work and is an employee of Amallis Consulting. M.S. received institutional grant funding from GlaxoSmithKline. K.J.M. has received grants from GlaxoSmithKline, Siemens Healthineers, and Profound Medical. M.A.J. has received grants from GlaxoSmithKline, the National Institutes of Health, and the National Cancer Institute. R.D., D.M., W.S. and P.P. are employees of ViiV Healthcare and own stock in GlaxoSmithKline. C.W.H. received grant funding from, and has served on advisory boards for, ViiV Healthcare and GlaxoSmithKline.
Figures


Comment in
-
Pharmacokinetically guided dosing to improve the efficacy of brigatinib in non-small cell lung cancer patients.Br J Clin Pharmacol. 2022 Aug;88(8):3920-3921. doi: 10.1111/bcp.15321. Epub 2022 Mar 29. Br J Clin Pharmacol. 2022. PMID: 35350084 No abstract available.
References
-
- Andrews CD, Heneine W. Cabotegravir long‐acting for HIV‐1 prevention. Curr Opin HIV AIDS. 2015;10(4):258‐263. - PubMed
-
- Radzio J, Spreen W, Yueh YL, et al. The long‐acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra5. - PubMed
-
- Saenger PH, Mejia‐Corletto J. Long‐acting growth hormone: an update. Endocr Dev. 2016;30:79‐97. - PubMed
-
- Wright JC, Burgess DJ. Long Acting Injections and Implants. New York: Springer; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

