Safety, pharmacokinetics and quantitative EEG modulation of TAK-071, a novel muscarinic M1 receptor positive allosteric modulator, in healthy subjects
- PMID: 34240455
- PMCID: PMC9291057
- DOI: 10.1111/bcp.14975
Safety, pharmacokinetics and quantitative EEG modulation of TAK-071, a novel muscarinic M1 receptor positive allosteric modulator, in healthy subjects
Abstract
Aims: TAK-071 is a muscarinic M1 receptor positive allosteric modulator designed to have low cooperativity with acetylcholine. This was a first-in-human study to evaluate the safety, pharmacokinetics, and pharmacodynamics of TAK-071.
Methods: TAK-071 was administered as single and multiple doses in a randomized, double-blind, placebo-controlled, parallel-group design in healthy volunteers alone and in combination with donepezil. Laboratory, electrocardiogram (ECG) and electroencephalogram (EEG) evaluations were performed. Cerebrospinal fluid and blood samples were taken to evaluate the pharmacokinetics (PK), relative bioavailability and food effect.
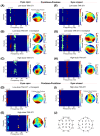
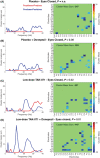
Results: TAK-071 was safe and well tolerated, and no deaths or serious adverse events occurred. TAK-071 demonstrated a long mean (% coefficient of variation) half-life of 46.3 (25.2%) to 60.5 (51.5%) hours and excellent brain penetration following oral dosing. Coadministration with donepezil had no impact on the PK of either drug. There was no food effect on systemic exposure. Quantitative EEG analysis revealed that TAK-071 40-80 mg increased power in the 7-9 Hz range in the posterior electrode group with eyes open and 120-160 mg doses increased power in the 16-18 Hz range and reduced power in the 2-4 Hz range in central-posterior areas with eyes open and eyes closed. Functional connectivity was significantly reduced after TAK-071 at high doses and was enhanced with coadministration of donepezil under the eyes-closed condition.
Conclusions: PK and safety profiles of TAK-071 were favorable, including those exceeding expected pharmacologically active doses based on preclinical data. When administered without donepezil TAK-071 was largely free of cholinergic adverse effects. Further clinical evaluation of TAK-071 is warranted.
Keywords: Alzheimer's disease; Parkinson's disease; electrophysiology; pharmacokinetic-pharmacodynamic; phase I.
© 2021 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
W.Y., D.L.B., P.K., D.V., L.R. and A.A.S. are employees of Takeda Pharmaceuticals. F.M. was a paid consultant to Takeda through Signal Insight, LLC, and was an employee of Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Figures



References
-
- Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiol Aging. 2000;21(1):11‐17. - PubMed
-
- Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. 2011;26(14):2496‐2503. - PubMed
-
- Davoren JE, Garnsey M, Pettersen B, et al. Design and synthesis of gamma‐ and delta‐lactam M1 positive allosteric modulators (PAMs): Convulsion and cholinergic toxicity of an M1‐selective PAM with weak agonist activity. J Med Chem. 2017;60(15):6649‐6663. - PubMed
-
- Davoren JE, Lee CW, Garnsey M, et al. Discovery of the potent and selective M1 PAM‐agonist N‐[(3R,4S)‐3‐hydroxytetrahydro‐2H‐pyran‐4‐yl]‐5‐methyl‐4‐[4‐(1,3‐thiazol‐4‐yl)ben zyl]pyridine‐2‐carboxamide (PF‐06767832): Evaluation of efficacy and cholinergic side effects. J Med Chem. 2016;59:6313‐6328. 10.1021/acs.jmedchem.6b00544 - DOI - PubMed
-
- Kurimoto E, Matsuda S, Shimizu Y, et al. An approach to discovering novel muscarinic M1 receptor positive allosteric modulators with potent cognitive improvement and minimized gastrointestinal dysfunction. J Pharmacol Exp Ther. 2018;364(1):28‐37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

