Multicompartmental pharmacokinetic evaluation of long-acting cabotegravir in healthy adults for HIV preexposure prophylaxis
- PMID: 34240467
- PMCID: PMC9290068
- DOI: 10.1111/bcp.14980
Multicompartmental pharmacokinetic evaluation of long-acting cabotegravir in healthy adults for HIV preexposure prophylaxis
Abstract
Aims: Cabotegravir is an integrase strand transfer inhibitor in clinical development as long-acting (LA) injectable HIV preexposure prophylaxis.
Methods: This phase I study assessed pharmacokinetics of cabotegravir in plasma and anatomical sites associated with sexual HIV-1 transmission after repeated oral and single intramuscular (IM) LA dosing in healthy adults. Following a 28-day oral lead-in period of cabotegravir 30 mg and a washout period of 14-42 days, participants were administered a single ultrasound-guided gluteal IM cabotegravir LA 600-mg injection. The study objective was to characterize cabotegravir concentrations in plasma, cervical, vaginal and rectal tissues, and cervicovaginal and rectal fluids and up to Week 12 after IM injection.
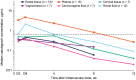
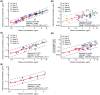
Results: Nineteen participants enrolled and 16 completed the study through Week 52. Cabotegravir was detected in plasma and all tissues and fluids. Median plasma cabotegravir concentrations exceeded the in vitro protein-adjusted 90% maximal inhibitory concentration through Week 12. Median tissue- and fluid-to-plasma cabotegravir concentration ratios across all visits were 0.32 for rectal fluid and 0.08-0.16 for other tissues and fluids. Adjusted R2 coefficients between cabotegravir concentrations in plasma and cervical, vaginal and rectal tissues were 0.78, 0.79 and 0.90, respectively. Injection-site reactions were common (88% of participants) and were mostly grade 1 in intensity (82%). Two participants reported 11 non-drug-related serious adverse events.
Conclusion: Concentrations of cabotegravir in tissues and fluids were proportional to plasma over time, with strong correlations between tissue and plasma concentrations. Cabotegravir LA tissue-to-plasma ratios may be important for understanding its use as preexposure prophylaxis.
Keywords: HIV/AIDS; antiretrovirals; pharmacokinetic-pharmacodynamic.
© 2021 ViiV Healthcare. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
J.S.S., K.B., K.H. and S.L.F. are employees of and own stock in GlaxoSmithKline. E.D.W. received institutional research funding from ViiV Healthcare and GlaxoSmithKline. S.E. has nothing to disclose. E.F. received grants from ViiV Healthcare and GlaxoSmithKline. S.R. received grants from GlaxoSmithKline, Merck and Gilead and consultant fees from Novimab. M.A.M. received grants from ViiV Healthcare and GlaxoSmithKline and the National Institutes of Health and research support from Gilead and Merck. R.D.A., W.S. and P.P. are employees of ViiV Healthcare and own stock in GlaxoSmithKline. Y.L. is an employee of Precision Biosciences and owns stock in GlaxoSmithKline. C.H. received grants from ViiV Healthcare, GlaxoSmithKline, Merck, Gilead and the National Institutes of Health; personal fees from Merck, ViiV Healthcare and GlaxoSmithKline; and nonfinancial support from Gilead. D.M. was an employee of ViiV Healthcare and may have owned stock in GlaxoSmithKline at the time of the study.
Figures
References
-
- UNAIDS UNAIDS Data 2020. www.unaids.org/en/resources/documents/2020/unaids-data. Accessed August 18, 2020.
-
- UNAIDS 90–90‐90: An Ambitious Treatment Target to Help End the AIDS Epidemic. www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed August 18, 2020.
-
- Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321(5):451‐452. - PubMed
-
- AIDSinfo The Basics of HIV Prevention. https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/20/48/the-ba.... Accessed July 29, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

