Nanopore metagenomic sequencing of influenza virus directly from respiratory samples: diagnosis, drug resistance and nosocomial transmission, United Kingdom, 2018/19 influenza season
- PMID: 34240696
- PMCID: PMC8268652
- DOI: 10.2807/1560-7917.ES.2021.26.27.2000004
Nanopore metagenomic sequencing of influenza virus directly from respiratory samples: diagnosis, drug resistance and nosocomial transmission, United Kingdom, 2018/19 influenza season
Abstract
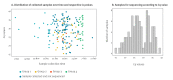
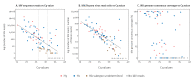
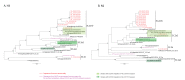
BackgroundInfluenza virus presents a considerable challenge to public health by causing seasonal epidemics and occasional pandemics. Nanopore metagenomic sequencing has the potential to be deployed for near-patient testing, providing rapid infection diagnosis, rationalising antimicrobial therapy, and supporting infection-control interventions.AimTo evaluate the applicability of this sequencing approach as a routine laboratory test for influenza in clinical settings.MethodsWe conducted Oxford Nanopore Technologies (Oxford, United Kingdom (UK)) metagenomic sequencing for 180 respiratory samples from a UK hospital during the 2018/19 influenza season, and compared results to routine molecular diagnostic standards (Xpert Xpress Flu/RSV assay; BioFire FilmArray Respiratory Panel 2 assay). We investigated drug resistance, genetic diversity, and nosocomial transmission using influenza sequence data.ResultsCompared to standard testing, Nanopore metagenomic sequencing was 83% (75/90) sensitive and 93% (84/90) specific for detecting influenza A viruses. Of 59 samples with haemagglutinin subtype determined, 40 were H1 and 19 H3. We identified an influenza A(H3N2) genome encoding the oseltamivir resistance S331R mutation in neuraminidase, potentially associated with an emerging distinct intra-subtype reassortant. Whole genome phylogeny refuted suspicions of a transmission cluster in a ward, but identified two other clusters that likely reflected nosocomial transmission, associated with a predominant community-circulating strain. We also detected other potentially pathogenic viruses and bacteria from the metagenome.ConclusionNanopore metagenomic sequencing can detect the emergence of novel variants and drug resistance, providing timely insights into antimicrobial stewardship and vaccine design. Full genome generation can help investigate and manage nosocomial outbreaks.
Keywords: Nanopore; antiviral drug resistance; diagnosis; genetic diversity; influenza; metagenomics; nosocomial transmission; respiratory viruses.
Conflict of interest statement
Figures

References
-
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Global Seasonal Influenza-associated Mortality Collaborator Network . Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285-300. 10.1016/S0140-6736(17)33293-2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
