Emerging role of PARP-1 and PARthanatos in ischemic stroke
- PMID: 34241907
- PMCID: PMC9010225
- DOI: 10.1111/jnc.15464
Emerging role of PARP-1 and PARthanatos in ischemic stroke
Abstract
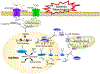
Cell death is a key feature of neurological diseases, including stroke and neurodegenerative disorders. Studies in a variety of ischemic/hypoxic mouse models demonstrate that poly(ADP-ribose) polymerase 1 (PARP-1)-dependent cell death, also named PARthanatos, plays a pivotal role in ischemic neuronal cell death and disease progress. PARthanatos has its unique triggers, processors, and executors that convey a highly orchestrated and programmed signaling cascade. In addition to its role in gene transcription, DNA damage repair, and energy homeostasis through PARylation of its various targets, PARP-1 activation in neuron and glia attributes to brain damage following ischemia/reperfusion. Pharmacological inhibition or genetic deletion of PARP-1 reduces infarct volume, eliminates inflammation, and improves recovery of neurological functions in stroke. Here, we reviewed the role of PARP-1 and PARthanatos in stroke and their therapeutic potential.
Keywords: NAD+; PARP-1; PARthanatos; oxidative stress; stroke.
© 2021 International Society for Neurochemistry.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
Figures


Similar articles
-
4'-O-methylbavachalcone alleviates ischemic stroke injury by inhibiting parthanatos and promoting SIRT3.Eur J Pharmacol. 2024 Jun 5;972:176557. doi: 10.1016/j.ejphar.2024.176557. Epub 2024 Apr 3. Eur J Pharmacol. 2024. PMID: 38574839
-
Poly (ADP-Ribose) polymerase 1 and parthanatos in neurological diseases: From pathogenesis to therapeutic opportunities.Neurobiol Dis. 2023 Oct 15;187:106314. doi: 10.1016/j.nbd.2023.106314. Epub 2023 Oct 1. Neurobiol Dis. 2023. PMID: 37783233 Review.
-
Crocetin antagonizes parthanatos in ischemic stroke via inhibiting NOX2 and preserving mitochondrial hexokinase-I.Cell Death Dis. 2023 Jan 21;14(1):50. doi: 10.1038/s41419-023-05581-x. Cell Death Dis. 2023. PMID: 36681688 Free PMC article.
-
Exploring the role of parthanatos in CNS injury: Molecular insights and therapeutic approaches.J Adv Res. 2025 Apr;70:271-286. doi: 10.1016/j.jare.2024.04.031. Epub 2024 May 3. J Adv Res. 2025. PMID: 38704090 Free PMC article. Review.
-
The dual role of poly(ADP-ribose) polymerase-1 in modulating parthanatos and autophagy under oxidative stress in rat cochlear marginal cells of the stria vascularis.Redox Biol. 2018 Apr;14:361-370. doi: 10.1016/j.redox.2017.10.002. Epub 2017 Oct 7. Redox Biol. 2018. PMID: 29049980 Free PMC article.
Cited by
-
Impact of anthocyanin on genetic stability in mammary adenocarcinoma-induced mice treated with methotrexate.Genes Nutr. 2022 May 5;17(1):6. doi: 10.1186/s12263-022-00709-8. Genes Nutr. 2022. PMID: 35513806 Free PMC article.
-
Mitochondria Related Cell Death Modalities and Disease.Front Cell Dev Biol. 2022 Mar 7;10:832356. doi: 10.3389/fcell.2022.832356. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35321239 Free PMC article. Review.
-
Molecular Targeting of Ischemic Stroke: The Promise of Naïve and Engineered Extracellular Vesicles.Pharmaceutics. 2024 Nov 21;16(12):1492. doi: 10.3390/pharmaceutics16121492. Pharmaceutics. 2024. PMID: 39771472 Free PMC article. Review.
-
Reverse Electron Transport at Mitochondrial Complex I in Ischemic Stroke, Aging, and Age-Related Diseases.Antioxidants (Basel). 2023 Apr 6;12(4):895. doi: 10.3390/antiox12040895. Antioxidants (Basel). 2023. PMID: 37107270 Free PMC article. Review.
-
PARP9 exacerbates apoptosis and neuroinflammation via the PI3K pathway in the thalamus and hippocampus and cognitive decline after cortical infarction.J Neuroinflammation. 2025 Feb 20;22(1):43. doi: 10.1186/s12974-025-03374-x. J Neuroinflammation. 2025. PMID: 39980030 Free PMC article.
References
-
- Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, & Endres M (2001). Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. International Journal of Molecular Medicine, 7, 255–260. 10.3892/ijmm.7.3.255 - DOI - PubMed
-
- Aberle L, Krüger A, Reber JM, Lippmann M, Hufnagel M, Schmalz M, Trussina IREA, Schlesiger S, Zubel T, Schütz K, Marx A, Hartwig A, Ferrando-May E, Bürkle A, & Mangerich A (2020). PARP1 catalytic variants reveal branching and chain length-specific functions of poly(ADP-ribose) in cellular physiology and stress response. Nucleic Acids Research, 48, 10015–10033. 10.1093/nar/gkaa590 - DOI - PMC - PubMed
-
- Andrabi SA, Kang HC, Haince J-F, Lee Y-I, Zhang J, Chi Z, West AB, Koehler RC, Poirier GG, Dawson TM, & Dawson VL (2011). Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nature Medicine, 17, 692–699. 10.1038/nm.2387 - DOI - PMC - PubMed
-
- Andrabi SA, Kim NS, Yu S-W, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, & Dawson TM (2006). Poly(ADP-ribose) (PAR) polymer is a death signal. Proceedings of the National Academy of Sciences, 103, 18308–18313. 10.1073/pnas.0606526103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

