Polymer-free Biolimus-A9 coated thin strut stents for patients at high bleeding risk 1-year results from the LEADERS FREE III study
- PMID: 34241947
- PMCID: PMC9544800
- DOI: 10.1002/ccd.29869
Polymer-free Biolimus-A9 coated thin strut stents for patients at high bleeding risk 1-year results from the LEADERS FREE III study
Abstract
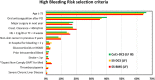
Background: In patients at high bleeding risk (HBR), the LEADERS FREE (LF) trial established the safety and efficacy of a polymer-free drug coated (Biolimus-A9) stainless steel stent (SS-DCS) with 30 days of dual antiplatelet treatment (DAPT). In LEADERS FREE III, we studied a new cobalt-chromium thin-strut stent (CoCr-DCS) in HBR patients.
Methods: The CoCr-DCS shares all of the design features of the SS-DCS but has a CoCr stent platform with strut thickness of 84-88 μm. The primary safety endpoint was a composite of cardiac death, myocardial infarction (MI), and definite/probable stent thrombosis. The primary efficacy endpoint was clinically indicated target lesion revascularization. Outcomes were compared to those of LF (non-inferiority to SS-DCS for safety and superiority to SS-BMS for efficacy). Additional propensity-matched comparisons were performed to account for baseline differences.
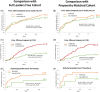
Results: We recruited 401 HBR patients using identical criteria to the LF trial. At 1 year, the primary safety endpoint was reached by 31/401 (8.0%) of patients treated with the CoCr-DCS versus 35/401 (8.9%) for the propensity-matched cohort (HR: 0.89, [0.55-1.44], p < 0.001 for non-inferiority, 0.62 for superiority). The efficacy endpoint was reached by 16/401 (4.2%) of CoCr-DCS patients versus 41/401 (10.6%) in the propensity-matched cohort (HR: 0.4 [0.2:0.7]) (p = 0.007 for superiority). There was no statistical difference between CoCr-DCS and SS-DCS in terms of efficacy (HR: 1.46 [0.68-3.15], p = 0.33).
Conclusions: The new thin-strut CoCr-DCS proved non-inferior to the SS-DCS for safety, and superior to the BMS for efficacy in HBR patients treated with 30 days of DAPT.
Keywords: CLIN-clinical trials; DES-stent; PCI-percutaneous coronary intervention (PCI); drug eluting.
© 2021 The Authors. Catheterization and Cardiovascular Interventions published by Wiley Periodicals LLC.
Conflict of interest statement
Hans‐Peter Stoll, and Sara Sadozai Slama are employees of the trial sponsor (Biosensors, Morges Switzerland). Diana Schütte and Philip Urban are consultants to Biosensors. Philip Urban and Philippe Garot are shareholders of CERC, the CRO conducting the trial, Philip Urban is a shareholder of MedAlliance (Nyon, Switzerland). The other authors have no conflict of interest to report.
Figures
References
-
- Urban P, Abizaid A, Chevalier B, et al. Rationale and design of the Leaders free trial: a randomized double‐blind comparison of the BioFreedom drug‐coated stent vs the Gazelle bare metal stent in patients at high bleding risk using a short (1 month) course of dual antiplatelet therapy. Am Heart J. 2013;165:7004‐7009. 10.1016/j.ahj.2013.01.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

