Epidemiology and disease burden of sickle cell disease in France: A descriptive study based on a French nationwide claim database
- PMID: 34242255
- PMCID: PMC8270152
- DOI: 10.1371/journal.pone.0253986
Epidemiology and disease burden of sickle cell disease in France: A descriptive study based on a French nationwide claim database
Abstract
Context: Sickle cell disease (SCD) is a severe hematological disorder. The most common acute complication of SCD is vaso-occlusive crisis (VOC), but SCD is a systemic disease potentially involving all organs. SCD prevalence estimates rely mostly on extrapolations from incidence-based newborn screening programs, although recent improvements in survival may have led to an increase in prevalence, and immigration could account for a substantial number of prevalent patients in Europe. The primary objective of this study was to estimate SCD prevalence in France.
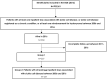
Methods: A cross-sectional observational study was conducted using a representative sample of national health insurance data. SCD patients followed up in France between 2006 and 2011 were captured through hydroxyurea reimbursement and with the International Classification of Diseases (ICD-10) SCD specific code D570.1.2, excluding code D573 (which corresponds to sickle cell trait (SCT)). Nevertheless, we assumed that ICD-10 diagnosis coding for inpatient stays could be imperfect, with the possibility of SCT being miscoded as SCD. Therefore, prevalence was analyzed in two groups of patients [with at least one (G1) or two (G2) inpatient stay] based on the number of SCD-related inpatient stays in the six-year study period, assuming that SCT patients are rarely rehospitalized compared to SCD. The prevalence of SCD in the sample, which was considered to be representative of the French population, was then extrapolated to the general population. The rate of vaso-occlusive crisis (VOC) events was estimated based on hospitalizations, emergencies, opioid reimbursements, transfusions, and sick leave.
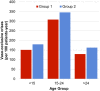
Results: Based on the number of patients identified for G1 and G2, the 2016 French prevalence was estimated to be between 48.6 per 100,000 (G1) or 32,400 patients and 29.7 per 100,000 (G2) or 19,800 patients. An average of 1.51 VOC events per year were identified, with an increase frequency of 15 to 24 years of age. The average annual number of hospitalizations was between 0.70 (G1) and 1.11 (G2) per patient. Intensive care was observed in 7.6% of VOC-related hospitalizations. Fewer than 34% of SCD patients in our sample received hydroxyurea at any point in their follow-up. The annual average cost of SCD care is €5,528.70 (G1) to €6,643.80 (G2), with most costs arising from hospitalization and lab testing.
Conclusion: Our study estimates SCD prevalence in France at between 19,800 and 32,400 patients in 2016, higher than previously published. This study highlights the significant disease burden associated with vaso-occlusive events.
Conflict of interest statement
JBA reports honoraria and consultancy/expert testimony for Novartis and Pfizer. MM reports honoraria and consultancy/expert testimony for Novartis and Addmedica, and Board participation for BlueBirdBio; MEJ reports honoraria and consultancy/expert testimony for Novartis and Pfizer; AH reports honoraria and consultancy/expert testimony for Novartis and Addmedica, and Board participation for BlueBirdBio. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Ware R.E., et al.., Sickle cell disease. The Lancet, 2017. 390(10091): p. 311–323. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

