Sperm-binding regions on bovine egg zona pellucida glycoprotein ZP4 studied in a solid supported form on plastic plate
- PMID: 34242308
- PMCID: PMC8270413
- DOI: 10.1371/journal.pone.0254234
Sperm-binding regions on bovine egg zona pellucida glycoprotein ZP4 studied in a solid supported form on plastic plate
Abstract
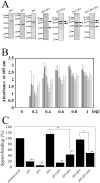
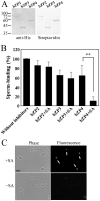
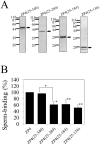
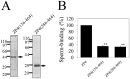
The zona pellucida (ZP) is a transparent envelope that surrounds the mammalian oocyte and mediates species-selective sperm-oocyte interactions. The bovine ZP consists of the glycoproteins ZP2, ZP3, and ZP4. Sperm-binding mechanisms of the bovine ZP are not yet fully elucidated. In a previous report, we established the expression system of bovine ZP glycoproteins using Sf9 insect cells and found that the ZP3/ZP4 heterocomplex inhibits the binding of sperm to the ZP in a competitive inhibition assay, while ZP2, ZP3, ZP4, the ZP2/ZP3 complex, and the ZP2/ZP4 complex do not exhibit this activity. Here, we show that bovine sperm binds to plastic plates coated with ZP4 in the absence of ZP3. We made a series of ZP4 deletion mutants to study the sperm-binding sites. The N-terminal region, Lys-25 to Asp-136, and the middle region, Ser-290 to Lys-340, of ZP4 exhibit sperm-binding activity. These results suggest that among the three components of bovine ZP glycoproteins, ZP4 contains the major potential sperm-binding sites, and the formation of a multivalent complex is necessary for the sperm-binding activity of ZP4.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Hinge Region of Bovine Zona Pellucida Glycoprotein ZP3 Is Involved in the Formation of the Sperm-Binding Active ZP3/ZP4 Complex.Biomolecules. 2015 Nov 23;5(4):3339-53. doi: 10.3390/biom5043339. Biomolecules. 2015. PMID: 26610590 Free PMC article.
-
Porcine zona pellucida glycoprotein ZP4 is responsible for the sperm-binding activity of the ZP3/ZP4 complex.Zygote. 2012 Nov;20(4):389-97. doi: 10.1017/S0967199411000608. Epub 2011 Oct 6. Zygote. 2012. PMID: 22008510
-
Recombinant bovine zona pellucida glycoproteins ZP3 and ZP4 coexpressed in Sf9 cells form a sperm-binding active hetero-complex.FEBS J. 2007 Oct;274(20):5390-405. doi: 10.1111/j.1742-4658.2007.06065.x. Epub 2007 Sep 26. FEBS J. 2007. PMID: 17894824
-
Mammalian zona pellucida glycoproteins: structure and function during fertilization.Cell Tissue Res. 2012 Sep;349(3):665-78. doi: 10.1007/s00441-011-1319-y. Cell Tissue Res. 2012. PMID: 22298023 Review.
-
The Human Egg's Zona Pellucida.Curr Top Dev Biol. 2018;130:379-411. doi: 10.1016/bs.ctdb.2018.01.001. Epub 2018 Mar 6. Curr Top Dev Biol. 2018. PMID: 29853184 Review.
Cited by
-
Integrated multi-omics analyses reveals molecules governing sperm metabolism potentially influence bull fertility.Sci Rep. 2022 Jun 23;12(1):10692. doi: 10.1038/s41598-022-14589-w. Sci Rep. 2022. PMID: 35739152 Free PMC article.
-
N-Glycosylation Site in the Middle Region Is Involved in the Sperm-Binding Activity of Bovine Zona Pellucida Glycoproteins ZP3 and ZP4.Biomolecules. 2023 Nov 10;13(11):1636. doi: 10.3390/biom13111636. Biomolecules. 2023. PMID: 38002318 Free PMC article.
-
Genome-based analysis of the genetic pattern of black sheep in Qira sheep.BMC Genomics. 2025 Feb 6;26(1):114. doi: 10.1186/s12864-025-11233-5. BMC Genomics. 2025. PMID: 39915708 Free PMC article.
-
Advances in Molecular Biology and Immunology of Spermatozoa and Fertilization in Domestic Animals: Implications for Infertility and Assisted Reproduction.Curr Mol Med. 2025;25(2):167-186. doi: 10.2174/0115665240306965240802075331. Curr Mol Med. 2025. PMID: 39572916 Review.
-
Identification of Sperm-Binding Sites in the N-Terminal Domain of Bovine Egg Coat Glycoprotein ZP4.Int J Mol Sci. 2022 Jan 11;23(2):762. doi: 10.3390/ijms23020762. Int J Mol Sci. 2022. PMID: 35054946 Free PMC article.
References
-
- Yonezawa N. Posttranslational modifications of zona pellucida proteins. In: Sutovsky P, editor. Posttranslational Protein Modifications in the Reproductive System. Springer: Berlin: Germany; 2014;759: 111–140. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

