A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies
- PMID: 34242585
- PMCID: PMC8319123
- DOI: 10.1016/j.devcel.2021.06.013
A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies
Abstract
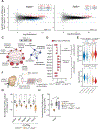
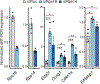
In ribosomopathies, perturbed expression of ribosome components leads to tissue-specific phenotypes. What accounts for such tissue-selective manifestations as a result of mutations in the ribosome, a ubiquitous cellular machine, has remained a mystery. Combining mouse genetics and in vivo ribosome profiling, we observe limb-patterning phenotypes in ribosomal protein (RP) haploinsufficient embryos, and we uncover selective translational changes of transcripts that controlling limb development. Surprisingly, both loss of p53, which is activated by RP haploinsufficiency, and augmented protein synthesis rescue these phenotypes. These findings are explained by the finding that p53 functions as a master regulator of protein synthesis, at least in part, through transcriptional activation of 4E-BP1. 4E-BP1, a key translational regulator, in turn, facilitates selective changes in the translatome downstream of p53, and this thereby explains how RP haploinsufficiency may elicit specificity to gene expression. These results provide an integrative model to help understand how in vivo tissue-specific phenotypes emerge in ribosomopathies.
Keywords: 4E-BP1; limb development; nucleolar stress; p53; ribosomal protein haploinsufficiency; ribosome profiling; ribosomopathy; translational control.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.R. is a shareholder of eFFECTOR Therapeutics and a member of its scientific advisory board.
Figures


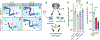


References
-
- Barna M, and Niswander L (2007). Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev. Cell 12, 931–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

