The histone H3.3 chaperone HIRA restrains erythroid-biased differentiation of adult hematopoietic stem cells
- PMID: 34242617
- PMCID: PMC8365107
- DOI: 10.1016/j.stemcr.2021.06.009
The histone H3.3 chaperone HIRA restrains erythroid-biased differentiation of adult hematopoietic stem cells
Abstract
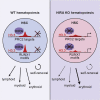
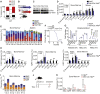
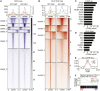
Histone variants contribute to the complexity of the chromatin landscape and play an integral role in defining DNA domains and regulating gene expression. The histone H3 variant H3.3 is incorporated into genic elements independent of DNA replication by its chaperone HIRA. Here we demonstrate that Hira is required for the self-renewal of adult hematopoietic stem cells (HSCs) and to restrain erythroid differentiation. Deletion of Hira led to rapid depletion of HSCs while differentiated hematopoietic cells remained largely unaffected. Depletion of HSCs after Hira deletion was accompanied by increased expression of bivalent and erythroid genes, which was exacerbated upon cell division and paralleled increased erythroid differentiation. Assessing H3.3 occupancy identified a subset of polycomb-repressed chromatin in HSCs that depends on HIRA to maintain the inaccessible, H3.3-occupied state for gene repression. HIRA-dependent H3.3 incorporation thus defines distinct repressive chromatin that represses erythroid differentiation of HSCs.
Keywords: Hira; differentiation; epigenetics; erythropoiesis; hematopoiesis; hematopoietic stem cell; histone H3.3; polycomb.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
J.F.M. is a founder of and owns shares in Yap Therapeutics. The other authors report no conflicts.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

