Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review
- PMID: 34243049
- PMCID: PMC8236076
- DOI: 10.1016/j.jiph.2021.06.013
Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review
Abstract
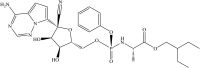
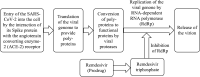
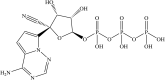
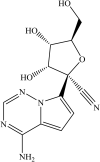
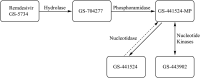
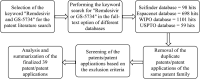
The development of remdesivir has been a breakthrough for COVID-19 treatment. It has been approved in about 50 countries, including Saudi Arabia, since 2020. The generic structure of remdesivir was first disclosed in 2009. This patent review summarizes the remdesivir based inventions to treat/prevent COVID-19 and other disorders from 2009 to May 16, 2021, emphasizing the patents related to medical and pharmaceutical sciences. The primary patents/patent applications of remdesivir are related to its compositions, new combinations with other therapeutic agents, delivery systems, and new indications. The inventive combinations have displayed synergistic effects against COVID-19, whereas the delivery systems/compositions have improved patient compliance. The inventions related to new indications of remdesivir to treat Ebola, hepatitis, idiopathic pulmonary fibrosis, diabetic nephropathy, and cardiovascular complications enhance its therapeutic area. Many new innovative combinations and delivery systems of remdesivir are anticipated to provide better treatment for COVID-19.
Keywords: COVID-19; Combination; Composition; Delivery system; New indication; Patent; Remdesivir.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- World Health Organization . 2021. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ [Accessed 16 May 2021]
-
- World Health Organization . 2021. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed 16 May 2021]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

