Selective Caries Removal in Permanent Teeth (SCRiPT) for the treatment of deep carious lesions: a randomised controlled clinical trial in primary care
- PMID: 34243733
- PMCID: PMC8267238
- DOI: 10.1186/s12903-021-01637-6
Selective Caries Removal in Permanent Teeth (SCRiPT) for the treatment of deep carious lesions: a randomised controlled clinical trial in primary care
Abstract
Background: Dental caries is one of the most prevalent non-communicable disease globally and can have serious health sequelae impacting negatively on quality of life. In the UK most adults experience dental caries during their lifetime and the 2009 Adult Dental Health Survey reported that 85% of adults have at least one dental restoration. Conservative removal of tooth tissue for both primary and secondary caries reduces the risk of failure due to tooth-restoration, complex fracture as well as remaining tooth surfaces being less vulnerable to further caries. However, despite its prevalence there is no consensus on how much caries to remove prior to placing a restoration to achieve optimal outcomes. Evidence for selective compared to complete or near-complete caries removal suggests there may be benefits for selective removal in sustaining tooth vitality, therefore avoiding abscess formation and pain, so eliminating the need for more complex and costly treatment or eventual tooth loss. However, the evidence is of low scientific quality and mainly gleaned from studies in primary teeth.
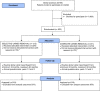
Method: This is a pragmatic, multi-centre, two-arm patient randomised controlled clinical trial including an internal pilot set in primary dental care in Scotland and England. Dental health professionals will recruit 623 participants over 12-years of age with deep carious lesions in their permanent posterior teeth. Participants will have a single tooth randomised to either the selective caries removal or complete caries removal treatment arm. Baseline measures and outcome data (during the 3-year follow-up period) will be assessed through clinical examination, patient questionnaires and NHS databases. A mixed-method process evaluation will complement the clinical and economic outcome evaluation and examine implementation, mechanisms of impact and context. The primary outcome at three years is sustained tooth vitality. The primary economic outcome is net benefit modelled over a lifetime horizon. Clinical secondary outcomes include pulp exposure, progession of caries, restoration failure; as well as patient-centred and economic outcomes.
Discussion: SCRiPT will provide evidence for the most clinically effective and cost-beneficial approach to managing deep carious lesions in permanent posterior teeth in primary care. This will support general dental practitioners, patients and policy makers in decision making. Trial Registration Trial registry: ISRCTN.
Trial registration number: ISRCTN76503940. Date of Registration: 30.10.2019. URL of trial registry record: https://www.isrctn.com/ISRCTN76503940?q=ISRCTN76503940%20&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic-search .
Keywords: Complete caries removal; Cost–benefit analysis; Minimally invasive dentistry; Oral-health-related quality of life; Partial caries removal; Patient-centred outcomes; Primary care; Randomised controlled trial; Selective caries removal; Willingness to pay.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing financial or other interests.
Figures
References
-
- World Health Organisation. Oral Health Country/Area Profile Project (CAPP). In: Oral Health Database. Sweeden: Malmo University; 2006.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical